| Pages:
1
..
3
4
5
6
7
..
23 |
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Magnesium Tricyano methanide Trinitro methanide
Quote: Originally posted by PHILOU Zrealone  |
http://www.sciencemadness.org/talk/viewthread.php?tid=3416#p...
Explosives that displays less H atoms (more insaturations) displays higher heat of explosions
...multiple bonding increases the energy per volume, and no or little water produced favourise
the best heat output.
N#C-C#N is one of the hottest burning stuff (4000°C vs 3100°C for H-C#C-H)...that's why
I think percyano compounds admixed with pernitrocompounds must be the best binary HE
of all times! It excludes H atoms and contains a lot of unsaturated energy rich bonds per volume.
But who wants to play with tetracyano methane and tetranitromethane |
Compounds made mostly of covalently bonded Nitrogen are not alone in storing energy
endothermically. In the Cyano ( Nitrile ) group we see that carbon , beside being a fuel ,
bonded to Nitrogen can also store energy endothermically. TricyanoTriazine the trimer
of Cyanogen hydrolysis readily, even cold is rapidly decomposed by water but it can
provide a framework on which to build. http://pubs.acs.org/doi/abs/10.1021/jo025833h
Chemical Reduction of 2,4,6-Tricyano-1,3,5-triazine and 1,3,5-Tricyanobenzene.
Formation of Novel 4,4‘,6,6‘-Tetracyano-2,2‘-bitriazine and Its Radical Anion
Carbon Nitrogen polymers , US patent 3057808
2,4,6-Tricyano-1,3,5-Triazine , US patent 5086172
Chemistry of Cyanogen Compounds
http://ia311327.us.archive.org/2/items/chemistrycyanog00will...
. . . . . . N == C-CΞN
. . . . . . / . . . . . \
NΞC-C . . . . . .N
. . . . . .\\ . . . . . //
. . . . . . N ---- C-CΞN
Replacing one Cyano group with a Trinitro methane would yield an impressive energetic
NΞC-CΞN + N2O4 might be induced to react gently producing NΞC-NO2 as a trimer thus _
. . . . . . N == C-NO2
. . . . . . / . . . . . \
NO2-C . . . . . .N
. . . . . .\\ . . . . . //
. . . . . . N ---- C-NO2
Synthesis And Chemistry Of Cyanogen
http://pubs.acs.org/doi/abs/10.1021/cr50029a003
It is also very possible a Tetrazine compound can be formed by different means
. . . . . N == C-NO2
. . . . ./ . . . . . .\
. . . .N . . . . . .N
. . . . .\\ . . . . . //
NO2-C ---- N
These will likely easily hydrolyze and be subject to derangement, but indicate areas for
further investigation.
There is much interest in compounding Trinitromethane usually in the form of a salt with
suitable reducing bases. Tricyanomethane has never been isolated and is known only by
it's salts and compounds. It's pKa of -5 means it is more acidic than sulfuric acid.
Trinitromethane is nearly as acidic so the prospect of mutual condensation is dismal.
Hexanitroethane can be considered two Trinitromethanes joined and is a stable molecule
so by example 1,1,1-Tricyano-2,2,2-Trinitroethane is not unthinkable. Cyanoform , HC(CN)3 ,
has an estimated ~ 800 KJ/mol heat of formation, Nitroform , HC(NO2)3 , is - 48.5 KJ/mol.
Synthesis and Thermochemistry of Tricyanomethyl and Other Polycyano Compounds
http://www.anl.gov/PCS/acsfuel/preprint archive/Files/09_1_DETROIT_04-65_0107.pdf
A compound consisting of both groups will be highly endothermic.
Utilizing the detonation calculator utility by forum member Engager , and entering
the nominal value of 1.6 for density and estimated 750 KJ/mol the projected
detonation pressure is 456 Kbar at 8680 m/s , by the Keshavarz method
entering density of 1.8 gives 577 Kbar at 9520 m/s
Tricyanomethane and Trinitromethane form ionic metal salts. A way in which the two
moieties might be compounded is by a solution of equal parts of Magnesium Tricyano -
methanide and Magnesium Trinitromethanide co-crystallized as a double salt , thus
Mg{C(CN)3}2 + Mg{C(NO2)3}2 => 2 Mg{C(CN)3C(NO2)3} => 2 MgO + 10 CO + 6 N2
If one is seeking a powerful " green " primary , one need look no further.
Relative solvation and strength of polycyano- and polynitromethanes in water
http://www3.interscience.wiley.com/journal/109741371/abstrac...
Trinitromethanide and Tricyanomethanide Salts Restricted to C, H, N, and 0 Atoms
http://handle.dtic.mil/100.2/ADA254523
redirects to _
http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA254523&Locati...
1. Superior Energetic Materials That Contain Coexisting Carbocations and Anions
2. Energetic Materials Restricted in Composition to C, H, N, and 0 Atoms
http://handle.dtic.mil/100.2/ADA275449
redirects to _
http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA275449&Locati...
Tricyanomethane (Cyanoform), Carbamyldicyanomethane, and Their Derivatives
http://pubs.acs.org/doi/abs/10.1021/jo01049a021
Depicted below are possible structures utilizing the Cyano ( Nitrile ) group
I do not have a notion how these could be made.
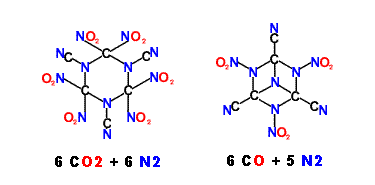
[Edited on 11-4-2010 by franklyn]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
| Quote: |
. . . . . . N == C-NO2
. . . . . . / . . . . . \
NO2-C . . . . . .N
. . . . . .\\ . . . . . //
. . . . . . N ---- C-NO2 |
This is essentially the oxidation product of melamine....a similar derivative of triazine exists, i.e. the cyanuric triazide (replace the NO2's by
N3's).
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Quote: Originally posted by franklyn  |
It is also very possible a Tetrazine compound can be formed by different means
. . . . . N == C-NO2
. . . . ./ . . . . . .\
. . . .N . . . . . .N
. . . . .\\ . . . . . //
NO2-C ---- N
These will likely easily hydrolyze and be subject to derangement, but indicate areas for
further investigation.
|
Dinitro-1,2,4,5-tetrazine was a much sought after target for a long time. The aminonitro dioxide has been made and found to be hydrolytically and
thermally unstable. Nice density though, at >1.9. Mind you, to make this uses hypofluorous acid...
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Tricyanomethane (Cyanoform), Carbamyldicyanomethane, and Their Derivatives
http://pubs.acs.org/doi/abs/10.1021/jo01049a021
related post
http://www.sciencemadness.org/talk/viewthread.php?tid=8544#p...
Crystal structures & thermal behavior of Tricyanomethanides ( reference citation )
http://www.znaturforsch.com/ab/v63b/63b0285.pdf
Alkali or Alkaline Tricyano methanides US patent 20100094040
.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
C2(NO2)2(N3)2 should be about just as powerful as tetranitrotetrahedrane. I think having a N atom in a pentagonal ring with positive charge and double
bonded to a side group C(NO2)2 would be a good way to add a powerful functional group to an energetic compound. Draw it on paper, it has some complex
resonance states that will provide high stability. This is analogous to nitroformate's stability C(NO2)3- cation, but with the group covalently bonded
to the main molecule.
I would like to add that some of the past posts have presented possible new molecules that have decomposition products with high energies of
formation. These heats of formation are inadequate to predict energetic performance. The C-N bonds are strong and will consume much energy in
breaking, futhermore I would expect the molecules, which are highly electron deficient because of lack of hydrogen atoms, to be too sensitive for
commercial use.
Also having a C=O bond is a waste. It does little to add power, and much to add sensitivity (for example there is an RDX compound with 2 of the H's
are replaced by an Oxygen. Why add an oxygen that is going to take up space when it won't add energy by oxidizing something? That is why I am not fond
of urea based compounds.
[Edited on 16-6-2010 by Anders Hoveland]
[Edited on 16-6-2010 by Anders Hoveland]
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
C=O generally gives a higher density than CH2. Higher density at the cost of a little energy is preferable over the reverse situation when high
brisance (VOD, Pcj) is the goal.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Higher density is only good if it packs in more energetic material. For example, ethyl nitrate is more dense than methyl nitrate, but also has a lower
det. velocity. Amine groups added onto a compound usually cause the molecules to pack closer together, whereas I am not sure about using a CO instead
of a CH2.
Other reasons amine groups are good is they are electron-donating, increasing stability. They can also add nitrogen, which can add power if the carbon
it is conncected to will get oxidized, otherwise an amine group can actually decrease the power of the energetic compound. An example is ethylene
1-amine, 2-nitrate. Also, if the energetic material is highly very energetic, the amine group, while having power, will actually reduce the "energetic
density", somewhat analagous to mixing TNT with RDX.
Another comment also, adding a CN will likely increase the temperature of the decomposition gases, but will not do much to add actual energy. Perhaps
some of the energy in an explosive is wasted on gases/ steam with high specific heats, that absorb much energy in boiling/ expanding.
[Edited on 17-6-2010 by Anders Hoveland]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Well, hey, if we're talking theoretical stuff, abet under research in this case, how about
Polymeric Nitrogen Stabilized on Carbon Nanotubes: A Highly Energetic, Green Explosive
Attachment: manning-polymeric-nitrogen.pdf (996kB)
This file has been downloaded 2547 times
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
WOW......19.73 km/s detonation velocity. Too bad it is not stable except under extreme conditions.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I saw one researcher prepare compounds with the ---CCSF5 group, a triple bond between the carbons. The SF5 is relatively inert, but the formation of
HF makes it potentially powerful, too bad the S--F bond is so strong.
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Higher density is only good if it packs in more energetic material.
|
Take a look at keto-RDX that you mentioned yourself; it has a higher density than RDX and a significantly higher VOD (a few percent better performance
than HMX). It is true that dinitroureas are often hydrolytically unstable, but that is a different matter. Generally speaking they exhibit very high
performance (in terms of brisance, not heaving power).
[Edited on 22-6-2010 by Microtek]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
A variation on the theme of compounding cyano and nitro salts is to combine
Dicyanamide and Dinitramine moieties co-crystallized as a double salt.
Magnesium dicyanamide plus Magnesium dinitramide , thus
Mg{N(CN)2}2 + Mg{N(NO2)2}2 => 2 Mg{N(CN)2.N(NO2)2} => 2 MgO + 2 CO2 + 2 CO + 6 N2
Very high density is to be expected , over 2 , with a high heat of explosiion.
.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
First off, dinitramide is only stable under fairly basic conditions. Mg(NO3)2 for example is acidic and a very strong dehydrating agent. It is not
possible to dry the hydrate by heating.
What about adding picric acid to neutralize the other amine in Hydrazinium perchlorate?
Keto-RDX is only more powerful because the O makes it more electron withdrawing and unstable, not because of density, even though it does in fact have
a higher "density".
In a good energetic compound one should aim for high energies of formation in the decomposition products, not trying to make everything as unstable as
possible.
By the way, I mixed acetone with a solution of bromine to make bromoacetone. Then made tri Bromoacetone-peroxide. Then absorbed it into benzene and
added AgNO3. Might have used AgClO4.
[Edited on 22-6-2010 by Anders Hoveland]
[Edited on 24-6-2010 by Anders Hoveland]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Anders Hoveland  | dinitramide is only stable under fairly basic conditions.
The dinitramide of Mg would be nearly impossible to make,
and would have to be kept away from any moisture. |
@ Anders Hoveland
If this reply sounds piqued it's because having otherwise exhibited competence
in your posts , you continue stating related mistaken assumptions after repeatedly
being corrected. Post too many wrong assertions and readers will be dismissive
of anything else you say. Read up on the item before commenting on something
you evidently have no knowledge of. The Magnesium salt is readily made by treating
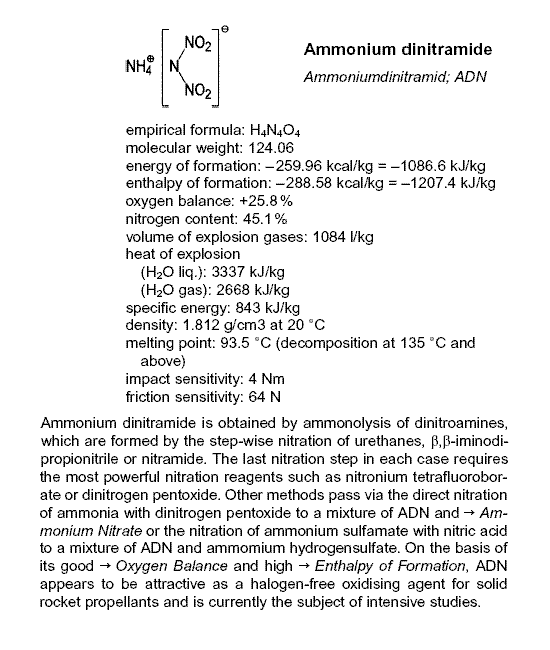
aqueous solution of Ammonium Dinitramide with Magnesium hydroxide.
Magnesium Dinitramide hexahydrate, Mg{N(NO2)2}2 • 6H20 then crystallizes out.
Disclosed here => http://www.springerlink.com/content/r44x114150263563
Because Dinitramidic acid decomposes , it is formed as the ammonium salt
which is commercially available.
While on the subject , some references _
Advances In Energetic Dinitramides
http://www.worldscibooks.com/chemistry/6618.html
Synthesis of Ammonium Dinitramide by Nitration of
Potassium and Ammonium Sulfamate
http://www.sid.ir/en/VEWSSID/J_pdf/84320080110.pdf
Detonation Properties and Reaction Rate Modeling of
Melt Cast Ammonium Dinitramide
http://www.intdetsymp.org/detsymp2002/PaperSubmit/FinalManus...
ADN – New Oxidizer for an Environmentally Friendly Smokeless Propellant
http://www.jatm.com.br/papers/vol1_n2/JATMv1n2_p153-160_ADN-...
New Insensitive High Explosives
http://preterhuman.net/texts/terrorism_and_pyrotechnics/expl...
Methods of Forming Dinitramide Salts
Patent US5198204
Process for Forming Dinitramide Salt by Reaction Between
Ammonia and a Nitronium Containing Compound
Patent US5316749
Process for Preparing Ammonium Dinitramide
Patent US5714714
Propellant Formulations Based on Dinitramide Salts
and Energetic Binders
Patent US5498303 and US5741998
Method of Preparing Dinitramidic Acid and Salts Thereof
Patent US5976483
Methods of Producing Salts of Dinitramidic Acid
Patent US20080226533
Preparation of Ammonium Dinitramide Crystals and
Energetic Composites Containing Them
Patent US20090090441
.
[Edited on 24-6-2010 by franklyn]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
The file was not found on the server in this URL.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
All right, I take it back; I was incorrect and could easily have checked to see whether the dintramide of magnesium existed. But only one reference
was really necessary to disprove me. You seemed to go at great, unnecessary lengths to show that the salt exists. I am not aware of other "mistaken
assumptions" that I have been corrected on that I continue to post. In just a few cases however, I have not found the evidence strong enough to change
my view. (for example, I am still of the opinion that Cl2O7 is unreactive in many cases, although it is prone to explode with just about anything)
Save your "piqued" responses for those other cases.
Microtek keeps going on about keto-RDX having a higher density and being more powerful. I do not disagree with that; I am just saying that in this
case higher density is not what is making it more powerful.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
John - the URL worked for me, just watch out for characters the board&browser stick in, and is the 1st hit if you search for the paper title.
http://www.jatm.com.br/papers/vol1_n2/JATMv1n2_p153-160_ADN-...
shortened
http://7.ly/3K
| Quote: | | Quote: | | You seemed to go at great, unnecessary lengths to show that the salt exists. |
...you continue stating related
mistaken assumptions after repeatedly
being corrected.
| Quote: | | ...I do not disagree with that; I am just saying that in this case higher density is not what is making it more powerful. |
|
There's a partial answer. And in response to your reason for doing as you did, I would say that you should - especially until you establish yourself
supply somewhat detailed reasons as to why your assertion is true, and if possible links or quotes from reference materials such as journal paper and
technical reports. And you can ask for similar from those with differing views, they need to be able to back up their assertions too.
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I don't know what you mean when you say that keto-RDX is more powerful because it is more unstable. Would you care to elaborate on what links
stability with brisance?
Also, high energy of formation of the decomposition products would be undesirable. I presume you meant to say the reverse.
In any case brisance is only weakly dependent on energy and much more strongly on density. You could take a look at the Kamlet-Jacobs equation for
predicting the VOD and Pcj of explosives.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I mean to say that some of the bonds in keto-RDX are weaker, because of the electron withdrawing effect of the oxygen, and the lack of two hydrogens
that are somewhat electron donating. keto-RDX is easy to make, just use urea and less formaldehyde. In my opinion, the added strength is not worth the
added sensitivity, if one is trying to get a high "power to instability ratio". Also, more unstable compounds are more brissant than more insensitive
compounds in small quantities. Consider Ethyl Perchlorate. I do not think it is a particularly strong explosive when compared to others in bulk, but
small quantities are capable of being almost instantly set off by a very small trigger, whereas a small sample of RDX would not go off together so
simultaneously because it has a higher threshold to detonate.
To illustrate what I mean about density, you could add an iodine onto the methyl group in TNT. That would increase density, but not add more power.
You could mix lead powder with your explosive. Obviously that would not make it more powerful. Adding non-functional groups might increase density,
but just dilutes the explosive.
[Edited on 25-6-2010 by Anders Hoveland]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
For comparison of CHNO energetics, density and heat of formation are both the driving factors with a subtle interplay between them. Naturally, the
incorporation of heavy atoms can lead to high densities (eq, lead azide) but heavy-atom energetics do not display high VODs.
If keto-rdx is less stable than RDX, said instability would be manifested as a more positive heat of formation.
k-RDX -41.9 KJ (Thermochimica Acta Volume 426, Issues 1-2, February 2005, Pages 53-60 )
RDX +58 KJ/mol (Journal of Hazardous Materials Volume 133, Issues 1-3, 20 May 2006, Pages 30-45 )
So we see keto RDX is a more stable compound, posessing stronger bonds, with an exothermic heat of formation
RDX on the other hand has a positive, endothermic heat of formation.
In the case of RDX vs keto RDX the higher detonation parameters of k-RDX is soley due to density, and is in spite of the more exothermic heat of
formation.
[Edited on 25-6-10 by The_Davster]
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Also, more unstable compounds are more brissant than more insensitive compounds in small quantities. Consider Ethyl Perchlorate. I do not think it is
a particularly strong explosive when compared to others in bulk, but small quantities are capable of being almost instantly set off by a very small
trigger, whereas a small sample of RDX would not go off together so simultaneously because it has a higher threshold to detonate.
|
It seems to me that you are confusing the concepts of stability and sensitivity. With regards to your statement about brisance of small amounts, that
is true but irrelevant; it is the difference between what we call primary and secondary explosives.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Primaries and secondaries are totally different.
With secondaries, we talk about Brisance and VoD and 'work potential' and so on.
With PRIMARIES, we discuss unequivocality, DDT threshold, etc.
Now, lets consider this. A primary goes in TINY amounts, and the good ones can do incredible work in such amounts... But they are unsuitable to use in
anything over, say, 1 gram.
1 gram TNT, RDX, etc aint much. Thats cos secondaries are for use in bigger amounts.
Density is useful cos shockwaves travel faster through more dense stuff, and cos you can pack more energy containing molecules into less volume.
Though tagging on useless shit like Iodine onto the Methyl in TNT is totally pointless cos it wont help the energy.
However, high density matter tends to allow us to pack more energy into smaller volume, hence better cos more more VoD. I am hungover right now and
cant explain but it is simple science. Microtek can explain better, as he is the 'God of Micro Charges'.
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Perhaps picric acid will neutralize the other amine in hydrazinium perchlorate, forming a double salt.
The picric acid would improve the oxygen balance and add stability.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Ok. Can you draw a nice picture to go with that, cos all I can think of is a co-ppt of Hydrazine-Picrate and Hydrazine Perchlorate. Though, I know
that Hydrazine-Azide and hydrazine-perchlorate, at OB neutral, is damn fine.
Or are you meaning something like in my shit attach picture?
I cant think straight, tired and stressed BTW. Sorry.
EDIT.
Pic wont fit or something. Fuck it. Basically it was
Picrate-(H2H4)-Perchlorate
[Edited on 30-6-2010 by -=HeX=-]
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Yes, (NO2)3PhO(-) (+)NH3NH3(+) ClO4(-)
Where there are N2H6 (+2) cations and a mix of picrate and perchlorate.
Now, let me throw in something to make it even MORE confusing.
Acetone forms a temporary condensation product with hydrazine; this is utilized in a modified Raschig synthesis (wikipedia).
Picric acid, as I have recently described in another post, likely will be able to react through a tautomeric ketone to condense with NH2OH... or with
NH2NH3(+) ! There is a diagram somwhere in "Quinone Explosives" topic.
Thus, may get the salt (NO2)3PhNNH3(+) ClO4(-)
This would be even more powerful, since it is the dehydrated form of the double salt I described.
Ph = phenyl obviously
|
|
|
| Pages:
1
..
3
4
5
6
7
..
23 |