| Pages:
1
..
8
9
10
11
12
..
23 |
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Azole-Based Energetic Salts reports a more reasonable 8786 m/s at 1.54 g/cc.
I don't think it should be compared with RDX in terms of brisance though, because the detonation pressure is much lower at 248 kbar. Still very
impressive for something so simple.
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
I recently found an alternativ and very easy synthetic route of / 2,2-dimethyl-5-hydroxymethyl-5-nitro-1 ,3-dioxane /
which is one precursor of the super explosiv BHDBT
It was dicussed one site before.
//
The way to obtain 2,2-dimethyl-5-hydroxymethyl-5-nitro-1,3-dioxane is illustrated by the following example.
To a mixture of 15.1 g (0.1 mol) of Tris-(hydroxymethyl)nitromethane and 50 ml of (0.68 mol) of acetone
with stirring and a temperature of 10°C sprinkled 5 g of phosphorus pentoxide. Stirred the reaction mass for
10 minutes, then poured into 100 ml saturated potassium carbonate solution with ice. Stir to dissolve the ice,
then the product is filtered, washed with cold water and dried in air. Gain of 11.5 g (71%) of
2,2-dimethyl-5-hydroxymethyl-5-nitro-1,3-dioxane with TPL 133-134°C.
http://russianpatents.com/patent/252/2529498.html
[Edited on 24-8-2015 by Mr.Greeenix]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Mr.Greeenix  | I recently found an alternativ and very easy synthetic route of / 2,2-dimethyl-5-hydroxymethyl-5-nitro-1 ,3-dioxane /
which is one precursor of the super explosiv BHDBT
It was dicussed one site before.
//
The way to obtain 2,2-dimethyl-5-hydroxymethyl-5-nitro-1,3-dioxane is illustrated by the following example.
To a mixture of 15.1 g (0.1 mol) of Tris-(hydroxymethyl)nitromethane and 50 ml of (0.68 mol) of acetone
with stirring and a temperature of 10°C sprinkled 5 g of phosphorus pentoxide. Stirred the reaction mass for
10 minutes, then poured into 100 ml saturated potassium carbonate solution with ice. Stir to dissolve the ice,
then the product is filtered, washed with cold water and dried in air. Gain of 11.5 g (71%) of
2,2-dimethyl-5-hydroxymethyl-5-nitro-1,3-dioxane with TPL 133-134°C.
http://russianpatents.com/patent/252/2529498.html
[Edited on 24-8-2015 by Mr.Greeenix] |
Very nice finding!
(CH3)2C(OCH2)2C(NO2)-CH2OH could also be halogenated to (CH3)2C(OCH2)2C(NO2)-CH2I;
then allowed to react with AgC(NO2)3 to yield (CH3)2C(OCH2)2C(NO2)-CH2-C(NO2)3;
then deprotected to yield (HOCH2)2C(NO2)-CH2-C(NO2)3 and finally nitrated to (O2NOCH2)2C(NO2)-CH2-C(NO2)3 (2-methylol-2,4,4,4-tetranitro-butanol
dinitrate = C5H6N6O14)
An energetic material with slightly positive OB:
C5H6N6O14 --> 5 CO2 + 3 H2O + 3 N2 + 1/2 O2
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
I found that:
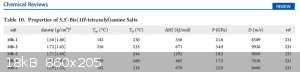
Attachment: Azole-Based Energetic Salts, Chemical Reviews.pdf (1.6MB)
This file has been downloaded 790 times
10b-2 : -> the ammonium salt of 5,5'-Bis(1H-tetrazolyl)amine
D (m/s): 9926m/s
P (GPa): 34.9
Has anybody an paper how to synthesis 5,5'-Bis(1H-tetrazolyl)amine ?=? 5-azido-1H-tetrazole??
/
/
Also I want to ask if someone can post this paper or just a synthesis of 5-Azido-1H-tetrazole (from 5-AT):
5-Azido-1H-tetrazole – Improved Synthesis, Crystal Structure and Sensitivity Data
http://onlinelibrary.wiley.com/doi/10.1002/zaac.200800003/ab...
Because the hydrazinium salt is quite interesting. Vod >9000
Attachment: ADA504339.pdf (138kB)
This file has been downloaded 597 times
/
/
I can't find this paper in somewhere here so I will just post it  It is easy to
synthes It is easy to
synthes
Attachment: Diaminouronium 5-nitriminotetrazolate.pdf (3.2MB)
This file has been downloaded 1131 times
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
http://en.cnki.com.cn/Article_en/CJFDTOTAL-BGXB200001006.htm
LU Ming jiu (Xi′an Modern chemistry Research Institute, Xi′an 710065,China)
The synthetic, structure, properties, and some applications of a new explosive 2,4,6,8,10,12 hexanitro 2,4,6,8,10,12 haxaaza tricyclo〔7.3.0.0 3.7
〕dodecane 5,11 dione(HHTDD) are described. The crystal density and detonation velocity of HHTDD were determined as 2.07 g/cm 3 and 9546
m/s.(ρ=1.995 g/cm 3) respectively. It is the first explosive with so high detonation velocity reported in references up to now.
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
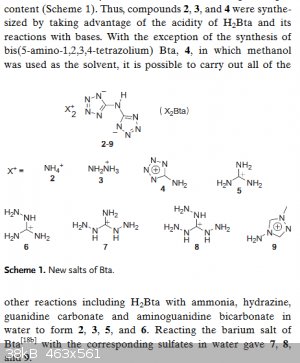
In my previous post I described the hydrazinium salt of 5,5'-Bis(1H-tetrazolyl)amine (not the ammonium salt. A fault in the document)
Synthetic route to 5,5'-Bis(1H-tetrazolyl)amine is decribed here:
| Quote: |
Synthesis of H2BTA•H2O (C2H3N9•H2O), (1)
As described in the literature[4], (Fig. 1) 4.45 g sodium dicyanamide (50 mmol) and 6.50 g sodium azide (100 mmol) were dissolved in 40 mL ethanol and
25 mL water, and then 75 mL of 2 M HCl was added dropwise over 4 h with stirring. The resulting mixture was refluxed for further 48 h. After cooling
to room temperature, 10 mL of 12 M concentrated HCl was added and then 60 white precipitates were filtered. The crude white solids were dissolved in
hot water, and then cooled to give white crystals 1.
IR (KBr): ν = 3455(s), 3030(s), 2932(s), 2857(s), 1651(vs), 1611(s), 1555(s), 1456(m), 1353(m), 1261(m), 1152(w), 1108(m), 1072(s), 1043(m), 1003(m),
903(m,br), 820(m), 740(m), 688(m), 509(m,br), 405(m) cm–1.
|
This is then allowed to react with hydrazine to yield the compound with vod of over 9900m/s
Attachment: 201310-123.pdf (336kB)
This file has been downloaded 862 times
Is there an other route to 5,5'-Bis(1H-tetrazolyl)amine? Pretty difficult
How to get sodium dicyanamide?
BTW does someone know how to syn. 5-Azido-1H-tetrazole? Maybe start from 5-Aminotetrazole?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Cyanogen bromide and NaN3 is the easiest.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Or hydrazino tetrazole and HNO2...
HN4C-NH-NH2 + HONO --> HN4C-N3 + 2 H2O
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Mr.Greeenix  | In my previous post I described the hydrazinium salt of 5,5'-Bis(1H-tetrazolyl)amine (not the ammonium salt. A fault in the document)
Synthetic route to 5,5'-Bis(1H-tetrazolyl)amine is decribed here:
| Quote: |
Synthesis of H2BTA•H2O (C2H3N9•H2O), (1)
As described in the literature[4], (Fig. 1) 4.45 g sodium dicyanamide (50 mmol) and 6.50 g sodium azide (100 mmol) were dissolved in 40 mL ethanol and
25 mL water, and then 75 mL of 2 M HCl was added dropwise over 4 h with stirring. The resulting mixture was refluxed for further 48 h. After cooling
to room temperature, 10 mL of 12 M concentrated HCl was added and then 60 white precipitates were filtered. The crude white solids were dissolved in
hot water, and then cooled to give white crystals 1.
IR (KBr): ν = 3455(s), 3030(s), 2932(s), 2857(s), 1651(vs), 1611(s), 1555(s), 1456(m), 1353(m), 1261(m), 1152(w), 1108(m), 1072(s), 1043(m), 1003(m),
903(m,br), 820(m), 740(m), 688(m), 509(m,br), 405(m) cm–1.
|
This is then allowed to react with hydrazine to yield the compound with vod of over 9900m/s
Is there an other route to 5,5'-Bis(1H-tetrazolyl)amine? Pretty difficult
How to get sodium dicyanamide?
BTW does someone know how to syn. 5-Azido-1H-tetrazole? Maybe start from 5-Aminotetrazole? |
This must be interesting to you...by Boffis in preparation folder:
Bis-(5-tetrazolyl)-amine
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
I found that.
Some very energetic salts based on 5-hydrazino-1H-tetrazole (5-Hydrazinotetrazole) (HTZ)
High energetic compounds with easy synthetic route. The only drawback is that you have to use the silver salt. I guess otherwise you will have some
impurities. The only common is silver nitrate
Attachment: Nitrogen-rich salts based on 5-hydrazino-1H-tetrazole.pdf (736kB)
This file has been downloaded 1008 times
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Glycolite
It was tested a little-known plastic explosive. The working title is Glycolite. It shows very good results. It contains 12% water, NaClO4 and
diethyleneglycol. This is the basic composition. Sensitizing are: aluminum and microballoons 1-3%. VoD is estimated to be 5,000 m / s or more.
According to the charge diameter and length of course. Details on Channel Laboratory of Liptakov........
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Laboratory of Liptakov  | It was tested a little-known plastic explosive. The working title is Glycolite. It shows very good results. It contains 12% water, NaClO4 and
diethyleneglycol. This is the basic composition. Sensitizing are: aluminum and microballoons 1-3%. VoD is estimated to be 5,000 m / s or more.
According to the charge diameter and length of course. Details on Channel Laboratory of Liptakov........ |
Very nice!
Glycerol could be used aswel with slight variation of the % for OB --> glycerolite 
This would be more viscous, denser and so VOD will be slighly better.
One may even think to a syrupy mix of erythritol and glycerol for even better density.
Or suggar syrup (corn syrup).
The use of NH4ClO4, HONH3ClO4 or N2H5ClO4 in the formulation will boost things up even more.
I wonder if NaClO3 what is more soluble and sensitive could be used instead of NaClO4...?
In principle nothing is against its use (no NH4, nor acid nor sulfur) in the original formulation.
[Edited on 8-11-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Glycolite
Huuh...NaClO3? It is interesting idea. Easy prepare from bleach. I hope, that NaClO3/DEG somebody will trying.
Liptakov
Hello! is there somebody for trying?
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Despite having a lower oxygen balance than perchlorates, chlorates tend to be much more unstable and sensitive to heat, friction, and shock. From my
experience, chlorates should be avoided due to the susceptibility for an accidental detonation, degradation and sensitivity over time.
[Edited on 11-9-2015 by Detonationology]
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Potassium 4,5-Bis(dinitromethyl)furoxanate: A Green Primary Explosive with a Positive Oxygen Balance
Chunlin He and Jean'ne M. Shreeve
Angew. Chem. Int. Ed. 2015, 54, early view
DOI: 10.1002/anie.201509209
Tetranitratoethane
Joerg Stierstorfer, Thomas M Klapoetke and Dennis Fischer
Chem. Commun., 2015, Accepted Manuscript
DOI: 10.1039/C5CC09010E
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Nicodem,
I like that!
I have requested those two articles in the reference section  
[Edited on 19-11-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Both articles are now available (a little after my request thanks to Solo in the reference section under pdf format (see link hereabove).
And hereunder are some infos from Franklyn on tetranitratoethane related methylene dinitrate or dinitratomethane (CH2(ONO2)2 geminal dinitrate ester
of dihydroxymethylene (formaldehyde hydrate aka CH2(OH)2 <=> CH2=O + H2O).
Methylene dinitrate
This is a new interesting family of compounds (H-(C(ONO2)2)n-H) that shows maybe promising HE properties, but they are easily hydrolysable although
the tendency seems to be reduced by increasing n.
Only two compounds of that familly are known up to now...
H-(C(ONO2)2)1-H = CH2(ONO2)2
H-(C(ONO2)2)2-H = (O2NO)2CH-CH(ONO2)2
When n increases so do:
-density
-Oxygen Balance (already positive!)
-VOD
-stability towards heat and hydrolysis
So maybe...
n=3
H-(C(ONO2)2)3-H = (O2NO)2CH-C(ONO2)2-CH(ONO2)2
hexanitratopropane
n=4
H-(C(ONO2)2)4-H = (O2NO)2CH-(C(ONO2)2-)2-CH(ONO2)2
octanitratobutane
n>4
...would prove to be even better on all those detonic and physico chemical aspects
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
MANGANIT
It was tested a new energetic material ( mixture) on KMnO4 based. No hygroscopic, cheap and easy get of oxidizer. But fuels are expensive and
difficult find for somebody. Basic mix is KMnO4 55, commerce nitrocellulose 12,5 to 17,5, shotgun double powder 12,5 - 17,5, aluminium Bright type
10-20 by parts of weight. Estimate VoD 2000 m/s by 1,25 g/cm3. At 10g charge from detonator No.8. Working name Manganit on Liptakov channel.... ...LL ...LL
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by Laboratory of Liptakov  | It was tested a new energetic material ( mixture) on KMnO4 based. No hygroscopic, cheap and easy get of oxidizer. But fuels are expensive and
difficult find for somebody. Basic mix is KMnO4 55, commerce nitrocellulose 12,5 to 17,5, shotgun double powder 12,5 - 17,5, aluminium Bright type
10-20 by parts of weight. Estimate VoD 2000 m/s by 1,25 g/cm3. At 10g charge from detonator No.8. Working name Manganit on Liptakov channel.... ...LL ...LL |
the VoD is very low !! what is the benefit of that ?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Laboratory of Liptakov  | It was tested a new energetic material ( mixture) on KMnO4 based. No hygroscopic, cheap and easy get of oxidizer. But fuels are expensive and
difficult find for somebody. Basic mix is KMnO4 55, commerce nitrocellulose 12,5 to 17,5, shotgun double powder 12,5 - 17,5, aluminium Bright type
10-20 by parts of weight. Estimate VoD 2000 m/s by 1,25 g/cm3. At 10g charge from detonator No.8. Working name Manganit on Liptakov channel.... ...LL ...LL |
Beware for friction sensitivity...
KMnO4 is hell a catalyst for sensitization...
Strange that you only reach 1,25g/cm3 with 55% KMnO4 at d= 2.7g/cm³ and 10-20% Aluminium also d=2.7g/cm³?
Maybe a lot of air trapped inside.
Beware for sternutative effect of ultrafine KMnO4 dust and also everything arround turning purple upon contact with moisture and finally brown from
oxydation into MnO2 spots very hard to get rid of!
@LL,
I like the comparative test flashpowder vs Manganex 
[Edited on 20-2-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Nice comparison Liptakov,
The patent below was posted many years back by quicksilver, might also be interesting to look at, especially considering the OTCness of terephtalic
acid.  The perchlorate version needs a lot of confinement to "detonate" from fuse
only, but they are nearly insensitive to normal stimuli. Don't think other oxidizers were included, maybe KMNO4 may be a nice tradeoff between fuse
sensitivity and confinement requirements. The perchlorate version needs a lot of confinement to "detonate" from fuse
only, but they are nearly insensitive to normal stimuli. Don't think other oxidizers were included, maybe KMNO4 may be a nice tradeoff between fuse
sensitivity and confinement requirements.
Pyrotechnic burster composition
US 6521064 B1
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Manganex / Manganit
This mixture has not an especially advantage. Only one. When you have a not another or better oxidizer, but have every all other compounds. This mix
working much better with NH4ClO4 and working good with KClO4, NH4NO3, best with TeACN and TeACP. But it is another energetic material, another
question, and nothing a new. Next thing, VoD is low. Well. But it is potassium permanganate. Is miracle, that sample was be detonated. And no only
deflagration, similarly as flash powder.
... ...LL Thanks for everybody of your feedback. https://www.youtube.com/watch?v=4lTIAhJLjmo ...LL Thanks for everybody of your feedback. https://www.youtube.com/watch?v=4lTIAhJLjmo
[Edited on 20-2-2016 by Laboratory of Liptakov]
|
|
|
octonitrocubane
Banned troll

Posts: 9
Registered: 17-3-2016
Member Is Offline
Mood: No Mood
|
|
My proffesor has synthesised hexamine where the nitro bonds are replaced with 'niflo' bonds. I have no idea how he did it. It seems unbelievably
sensitive - apparentely, it exploded in a shockproof container in a climate controlled atmosphere. He tamed one kilo of it, and ignited it with 0,5
grams ETN. It vapourised the metal at the centre and shredded the metal at the edges. Detonation velocity is around 9-10000 metres/second. If I can
find how to synthesise it, I will tell you.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by octonitrocubane  | | My proffesor has synthesised hexamine where the nitro bonds are replaced with 'niflo' bonds. I have no idea how he did it. It seems unbelievably
sensitive - apparentely, it exploded in a shockproof container in a climate controlled atmosphere. He tamed one kilo of it, and ignited it with 0,5
grams ETN. It vapourised the metal at the centre and shredded the metal at the edges. Detonation velocity is around 9-10000 metres/second. If I can
find how to synthesise it, I will tell you. |
Can you make a chemical drawing of the putative nitro-hexamine and niflo-hexamine? Maybe ask you teacher.
I'm sceptical about the veracity of the story...maybe your teacher told you a beautiful story...
1°) 1 kg of an explosive in a shockproof container is way too much for testing.
2°) synthetising 1 kg of an unknown sensitive explosive material is also way too much.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Lolololol
My teacher made and detonated a small atomic bomb in her jacket pocket yesterday.
No idea how how she managed to contain the half-megaton explosion using just Tweed and leather elbow patches ...
Maybe it was Harris Tweed.
|
|
|
| Pages:
1
..
8
9
10
11
12
..
23 |