happycamper723
Harmless

Posts: 10
Registered: 23-4-2012
Member Is Offline
Mood: Curious
|
|
Sulfuric Acid
I've learned from my last attempt that I should use a dry glass.
But here lies the question: What is the best way to make sulfuric acid???
Here are my priorities:
1. Cheap
2. Safe
3. Simple
4. Not time-consuming
5. Produces quality product
6. Everything else desirable (?)
Now i've looked over the sticky with like 40,000 views. I am not trying to produce large amounts of sulfuric acid, and I really don't know where to
find a lead chamber.
I would try simply bubbling SO2 into H2O2, but that produces a VERY dilute acid (boiling it down is far to dangerous for someone of my
limited knowledge and attention span).
I could try burning KNO3 and sulfur in the a steamy setting, but I don't know how I would set that up. I have an idea here of how I might do it...
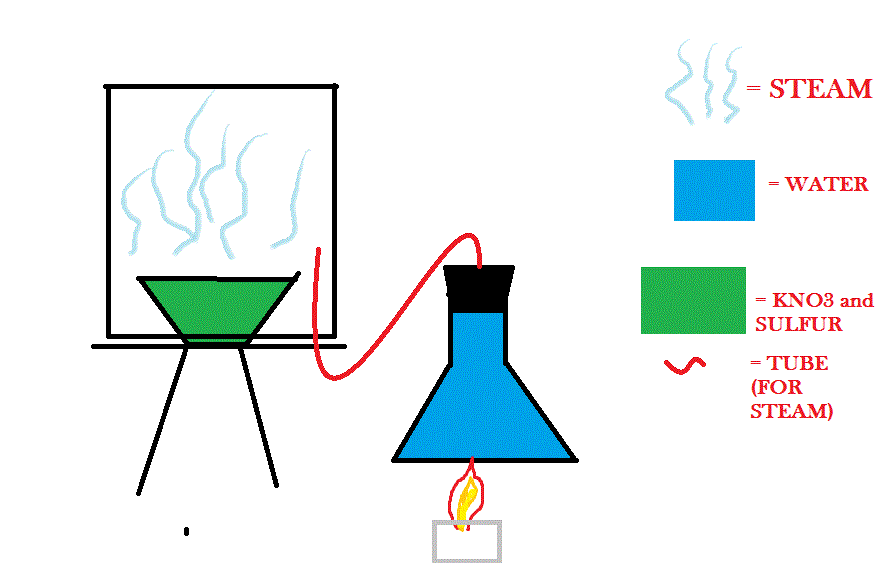
Will that work well?
[Edited on 26-4-2012 by happycamper723]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I would honestly recommend electrolysis of a transition metal sulfate.
For example, copper sulfate can do this easily, and is a relatively easily-obtained chemical (pottery supply, McLendon's, Ace, etc.)
When the liquid is not blue, all the copper sulfate has been electrolyzed, and this can be boiled down (if it turns blue again, repeat electrolysis).
This will give you some quantity of sulfuric acid.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by happycamper723  | | Now i've looked over the sticky with like 40,000 views. I am not trying to produce large amounts of sulfuric acid, and I really don't know where to
find a lead chamber. |
Read it through completely then, because you would have found out that certain
plastics can substitute for lead, and not exotic plastics.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Pass steam over Oxalic acid and MgSO4. Reaction:
MgSO4 + H2C2O4 --> MgC2O4 (s) + H2SO4
Relatively safe and cheap (depending on what deal you can get on Oxalic acid). Also, definitely simple, not time-consuming and capable of producing a
quality product.
There is a Sciencemadness thread on the uses of Oxalic acid (Link: http://www.sciencemadness.org/talk/viewthread.php?tid=18963 ) and one YouTube video on a synthesis (Link: http://www.youtube.com/watch?v=5cnqWepbVhY&feature=g-upl... ). Check it out.
[Edited on 27-4-2012 by AJKOER]
|
|
|
Pyridinium
Hazard to Others
  
Posts: 258
Registered: 18-5-2005
Location: USA
Member Is Offline
Mood: cupric
|
|
Quote: Originally posted by happycamper723  |
I would try simply bubbling SO2 into H2O2, but that produces a VERY dilute acid (boiling it down is far to dangerous for someone of my
limited knowledge and attention span). |
Not to pile on you, but the "limited knowledge and attention span" might be a good reason to stick with the very dilute H2SO4 until you build up the
level of knowledge (and patience) for handling stronger varieties.
That said, if you had something to catch the condensate beneath the steam chamber, it looks like the diagram might work. I don't like how much water
your flask shows there, but that probably wasn't intended. Also, obviously, you wouldn't heat the flask with a direct flame.
If you are set on doing it this way, consider doing it on as small a scale as possible (using small glassware). Even there, ventilation would be
extremely important.
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
http://www.youtube.com/watch?v=xHLeFSY_OJU&list=UUB4M84U...
Safe Way to do so
cheap way (if you have a fish pump)
Simple way
Ive got alot of complements for coming up with this idea 
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Another simple synthesis for H2SO4 I am planning on trying out:
1. Add fine Sulfur powder to H2O. Stir to make a suspension. If one has fresh colloidal Sulfur from, say, scrubbing H2S or another source, I would
speculated, given the large reactive surface area, that would be best for this synthesis.
2. Treat with Chlorine (see net reaction equation below for required quantity of 'reacted' Cl2).
3. Bubble air into the concentrated solution to remove unwanted HCl.
Chemistry:
3 Cl2 + 3 H2O <---> 3 HOCl + 3 HCl
S + 2 HOCl + H2O ---> H2SO3 + 2 HCl
H2SO3 + HOCl ---> H2SO4 + HCl
So, on net:
3 Cl2 + 4 H2O + S --> 6 HCl + H2SO4
So, 3 moles of Chlorine is required for each mole of Sulfur. Note the moles of water consumed possibly permitting the formation of concentrated acid.
Source:
Per Watts' Dictionary of Chemistry, Volume 2, Page 16, even dilute solution of HOCl can oxidize Sulfur all the way to H2SO4. To quote the relevant
section from Watts':
"Reactions.--1. HClOAq acts generally as an oxidiser; it easily parts with 0 while HClAq remains. Thus, As is rapidly oxidised with evolution of
light; P, S, Se, Br, I are converted to H3P04Aq, H2S04Aq, &c., even by dilute HClOAq; lower oxides or salts are converted into higher, e.g. SO2Aq
to H2SO4Aq, FeO to Fe203, As203Aq to As2O5Aq, FeS04Aq to Fe2(S04)3Aq, Fe2Cl6Aq, and Fe2O3, MnSO4Aq to MnO2; sulphides yield sulphates, c.g. H2SAq
gives" H2SO4,Aq and S; "
Link: http://books.google.com/books/reader?id=ijnPAAAAMAAJ&dq=...
[Edited on 30-4-2012 by AJKOER]
|
|
|
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
You got several ways to go one of the way is indeed the copper sulfate method I got a youtube video about the whole proces:
http://www.youtube.com/watch?v=vem4COJtihY
Another way could be by using sodium methabisulphate and HCl in an improvised gas generator which leads to 35% hydrogen peroxide.
You can also buy it as draincleaner which is already from a very high quality 98% pure not much contaminations but then you actually haven"t made it.
I am sure there are still alot of other ways but those are just the ways where I am familiar with if you would have any questions let me know!
~CrEaTiVePyroScience
|
|
|
nerdalert226
Harmless

Posts: 14
Registered: 22-12-2011
Location: United States
Member Is Offline
Mood: Bose-Einstien Condensate
|
|
that may work but, where will your H2SO4 deposit in the box?
|
|
|
Pyridinium
Hazard to Others
  
Posts: 258
Registered: 18-5-2005
Location: USA
Member Is Offline
Mood: cupric
|
|
Just to mention a caution to this method ... couple people on here have tried this and found it goes rather exothermic (as in, boiling / spattering).
|
|
|