Cloner
Hazard to Others
  
Posts: 150
Registered: 7-12-2004
Member Is Offline
Mood: apocalyptic
|
|
debromination of bromocyclopentanes
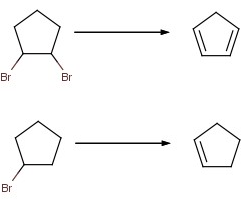
I want to create cyclopentadiene from bromocyclopentane.
While making bromocyclopentane, I also obtained some dibromocyclopentane isomers boiling at over 180 degrees.
Therefore, my strategy will be to heat the dibromo isomers with ethylene glycol and hydroxide (adding sodium hydroxide, or should I add sodium
metal?).
Of course, if I want a more controlled reaction (and more compound to work with) I will need to make 1,2-dibromocyclopentane from
monobromocyclopentane, using basically the same reaction.
Alternatively, I can imagine dehydrating cyclopentanol would also work.
What would be better, dehydrating the cyclopentanol or dehydrobrominating the bromide?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
React with solid NaOH dissolved in absolute alcohol (no water). That would certainly work in the second case.
In the first case (with two bromine atoms), you might get the formation of a triple bond instead (if the bromine atoms are vicinal to eachother, as
shown in the picture).
I would suggest that you could simplify your reaction, and avoid potential problems, by not using the isomer with vicinal bromines.
1,3-dibromocyclopentane would be better.
You could add sodium metal to the ethylene glycol (this would have to be done first), but this would probably be unnecessary.
You seem to be on the right track. Whichever route you choose, it depends on what precursors you have available to you. There are advantages and
disadvantages to both options.
|
|
|
Cloner
Hazard to Others
  
Posts: 150
Registered: 7-12-2004
Member Is Offline
Mood: apocalyptic
|
|
Thank you for replying. If a triple bond forms rather than a double bond, then my procedure is problematic: I started from cyclopentane. The monobromo
compound can be isolated easily. Removing hydrobromic then adding bromine would give 100% of the 1,2-dibromo isomer.
If I increase the bromine to cyclopentane ratio, a whole mess of isomers results, one of which is the 1,3-isomer.
Anyway, the heavy, high boiling fraction does react with sodium hydroxide and after steam distillation, it does discolor bromine water, meaning there
are double bonds present. Unfortunately, if there is cyclopentadiene at all, it isn't enough to show a clear boiling point.
I can use anhydrous alcohol. However, it seemed to me that heating the mixture would be a good thing, thus glycol. In fact, I was considering
microwaving the reaction mixture with glycol in a teflon pressure vessel (this reaction looks like one that can be done well with MW).
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
You won't get a triple bond in a ring that small - at least, not as a stable molecule. Triple bonds and the substituents at each end preferentially
form a linear, 4-atom segment. With only 5 carbons in the ring, there is a lot of ring strain in a cylclopentyne. 8 carbons is the smallest stable
hydrocarbon cycloalkyne, as far as I know.
Cyclopentadiene itself is not stable in pure form at room temperature - it dimerizes to form a Diels-Alder product, two double bonds from one molecule
react with one double bond of another.
|
|
|
Nicodem
|
Thread Moved
7-5-2012 at 08:46 |
Cloner
Hazard to Others
  
Posts: 150
Registered: 7-12-2004
Member Is Offline
Mood: apocalyptic
|
|
 of course, you are absolutely right on the strain part, I should have made the
transfer from structural formula to spatial formula myself. I know of the diels-alder reaction, after all, the normal way to make the compound is to
start with the adduct. However, the DA reaction is reversible and the balance is in favor of cyclopentadiene at higher temperatures. of course, you are absolutely right on the strain part, I should have made the
transfer from structural formula to spatial formula myself. I know of the diels-alder reaction, after all, the normal way to make the compound is to
start with the adduct. However, the DA reaction is reversible and the balance is in favor of cyclopentadiene at higher temperatures.
|
|
|