chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
N-alkylation of 4-piperidone
4-piperidone is the name given to 4-piperidone
monohydrochloride monohydrate which I believe is 4,4-piperidine diol HCl. It's not very reactive stuff. I have been trying to find an appropriate
solvent system to conduct N-alkylation using K2CO3 powder as the base. {A decade or so ago there was an internet hoax called the Siegfreid method of
making fentanyl wherein 4-piperidone was supposedly N-alkylated with ph-et-Br. It got mentioned in Microgram, probably to mislead would-be cooks.}
I've been playing around with keto-amines for awhile. Making them usually entails a Dieckman cyclization of an amine diester which is made from an
acrylate. One of the limitations is availability of the precursor acrylate. Several times over the years I beat my head against trying to make an
aryl-4-piperidone via alkylation of the 4-piperidone of commerce. I had just decided to attempt this again and was experimenting with every solvent
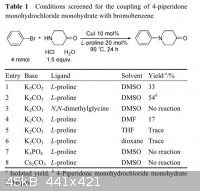
on my shelves. I did not try DMSO and was getting around to it after DMF when I ran across the attached paper. It made me a little more optimistic
and seemed a lot more straightforward than going through the methiodide. I am a little skeptical because the journal is Chinese and because the
authors claim using dmso as the solvent. They also claimed dissolving it in THF and although my notebook is at work I'm sure I tried that. I should
have tried dissolving the 4-piperidone in dmso before now and will tomorrow.
Attachment: 54336881-A-Facile-Synthesis-of-N-Aryl-Substituted-Piperidones-Chinese-Journal-of-Chemistry-2009-27-10-1995-2000.pdf (76kB)
This file has been downloaded 1207 times
[Edited on 1-7-2012 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
i think you would have more luck if you o-protect itt first as the ketal with ethylene glycol.
it has been done with the piperidinol too with no problems.
this is because it does'nt self condense the problem was the oxidation
anyway it went off without a hitch that way because the ketone was'nt self consdensing forming polymers as i told you so many times.
and you get the same results with o-protection.
there is a way to make ethyl acrylate using a tube furnace.
pyrolysis of ethyl lactate the new green solvent you see on the wal-mart shelves)
if i was'nt so lazy i would post references and data.
oh and if we talking about what i think the reason that shit is so boring is it's selectivity for mu you got to have ndma antagonism.
you see this with prodines and ketobemidones and things like dextromoramide.
but i have a theory here why don't you skip that altogether and just add phenylethylene oxide across that amine you would get an alpha hydroxylated
analog.
it would in turn be esterified but it would hydrolyze at a faster rate than the amide i would'nt bother though the ester just clads on more loopholes
unless esters were specificially mentioned i don't count on those people being as smart as everyone thinks they are.
[Edited on 2-7-2012 by jon]
[Edited on 2-7-2012 by jon]
[Edited on 2-7-2012 by jon]
[Edited on 2-7-2012 by jon]
[Edited on 2-7-2012 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I'm sure you have someone in mind and some process you were familiar with. You're probably well motivated too. Maybe just a little confused.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
yeah i'm talking about real world examples using the alcohol rather than the piperidone as the starting material.
illlustrating that it's oxygen is unreactive and the n-alkylation went smoothly.
in order to drive home the point o protection would make things much easier.
i have little interest in that besides the chemistry of it.
[Edited on 2-7-2012 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I'm sure you have a conversation with someone somewhere in mind. If I don't have a clue what you're talking about I'm sure it my loss.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
yeah a guy i met in jail.
oh speaking of that little stunt you pulled i made them like a bunch of amateurs, it was a nice try.
next time you drop a dime it costs 50 cents you might get better luck.
[Edited on 2-7-2012 by jon]
[Edited on 2-7-2012 by jon]
[Edited on 2-7-2012 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
I have worked with substituted piperidines for some time. One method for N-alkylated substrates is to react substituted 1,5-dichloropentanes with
substituted anilines. It requires some tweaking with reaction conditions but can be done. Tan D. Quach and Robert A. Batey have published a boronic
acid coupling approach in Org. Lett. DOI: 10.1021/ol035681s. Might be worth taking a look.
Why not use Buchwald-Hartwig amination? Requires some exotic ligands and palladium, but works like charm.
Caubère coupling might be a more amateur friendly approach. NaNH2/tBuONa, PhBr in THF at 50 °C overnight gave 74 % yield of the acetal protected
piperidone. This method was used in the total synthesis of (±)-Pumiliotoxin C (DOI: 10.1002/ejoc.200400846)
[Edited on 2-7-2012 by kavu]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by chemrox  | | I am a little skeptical because the journal is Chinese and because the authors claim using dmso as the solvent. They also claimed dissolving it in
THF and although my notebook is at work I'm sure I tried that. |
I'm not sure if you are aware that there is not a single example of N-alkylation in that article. There are described only Ullmann condensations of
4-piperidone with aryl halides. These obviously have nothing to do with N-alkylations of which you talk at the beginning and in the title.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Well you're right of course. They used aryl halides across the board. Good call. @kavu here's another paper I found in my library and wish I'd read
more carefully as well. I confirmed that the 4-piperidone Hcl hydrate is soluble in dmso. I haven't been able to tell if the solution is bubbling
due to CO2 evolution on addition of K2CO3 powder or if tiny bubbles result from stirring the carbonate in. The K2CO3 was purchased as K2CO3 "powder."
It looked pretty granular to me so I put it on the ball mill for a couple of days.
Attachment: Buchwald-Hartwig synth.pdf (214kB)
This file has been downloaded 3148 times
So here's what I'm wondering. I can make the ethyl ketal and use it as written in these papers. As soon as I satisfy myself that ph-et-piperidone
can be made from 4-piperidone Hcl hydrate and ph-et-Br I want to move on to substituted piperidones. I'm wondering if in say 2-methyl-4-piperidone
one needs the ketal at all or will the keto-amine react favorably with alkyl & aryl halides?
[Edited on 3-7-2012 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
no the keto amine reacts smoothly.
w/o protection.
[Edited on 5-7-2012 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|
Nightshader
Harmless

Posts: 2
Registered: 21-10-2012
Member Is Offline
Mood: No Mood
|
|
What do you think about the method presented by Gupta et. al. in their paper on scribd "A convinient one-pot synthesis of fentanyl"... easyliy
available by googling the term?
They use 1,2-dichloroethane as solvent and they perform a SN2(nucleophilic substitution) reaction of phenylacetaldehyde on the 4-piperidone and
afterwards reducing the double bond with a mild variation of NaBH4 called STAB (sodiumtriacetoxyborohydride) which is easily preparated from NaBH4 and
Acetic acid in toluene. The mentioned Triethylamine is a helping base in this reaction.
When you look at the end product´s yield you can guess that this reaction must have a high yield without touching the keto group.
For me this reaction makes perfect sense when you look at SN2´s mechanism....
|
|
|
Dr.Bob
International Hazard
    
Posts: 2659
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nightshader  | | They use 1,2-dichloroethane as solvent and they perform a SN2(nucleophilic substitution) reaction of phenylacetaldehyde on the 4-piperidone and
afterwards reducing the double bond with a mild variation of NaBH4 called STAB (sodiumtriacetoxyborohydride) which is easily preparated from NaBH4 and
Acetic acid in toluene. For me this reaction makes perfect sense when you look at SN2´s mechanism.... |
That is odd, since this is a reductive amination reaction, not an SN2 reaction. The phenylacetaldehyde forms the imine with the piperidine N, and
then is reduced by the NaBH(OAc)3 to form the tertiary amine. The preparation is a simple procedure, but finding all of the the starting materials
is not, given the obvious use for them.
|
|
|
Nightshader
Harmless

Posts: 2
Registered: 21-10-2012
Member Is Offline
Mood: No Mood
|
|
I know it is an reductive amination, but in progress of this I thought this would be a
nucleophilic substitution with formation of an enamine, where the double bond would be
reduced by the STAB.
Besides, all of the Ingredients for the whole process, even propionylchloride and 4-piperidone are readily
available to almost everyone here in the very centre of europe.
As the TO already said, 4-piperidone is sometimes confused with 4-piperidinediol-hydrate,
which is, according to erlenmeyer´s, the same.
To get off the drug thing, if the TO wants to make the aryl analog, he might be fine using BA.
[Edited on 22-10-2012 by Nightshader]
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
Lacking ketene
Quote: Originally posted by jon  |
there is a way to make ethyl acrylate using a tube furnace.
pyrolysis of ethyl lactate the new green solvent you see on the wal-mart shelves)
if i was'nt so lazy i would post references and data.
] |
Sorry, it's not that easy.
Attachment: Methyl Acrylate from LactateUS2417748.pdf (196kB)
This file has been downloaded 1567 times
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
yeah granted wiring pyrex tubing with nichrome and ataching it to a vairiac in line with n2 an addition funnel a bubbler can be precarious if the
glass has any localized variations in temperature to cause it to crack it would be a disaster that's because of ketene but the earlier methods look
safer have a look at this
i'm handy with metals as the elves are known to be it thing this would be safer even.
https://docs.google.com/viewer?a=v&q=cache:xNHAhU9gpQUJ:...
[Edited on 3-11-2012 by jon]
[Edited on 3-11-2012 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|