liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
Copper Acetate
When producing copper (II) acetate electrochemically with a copper cathode/acetic acid solution, are there any anode materials that will not reduce
the copper acetate back into copper and acid? If not, how could I layout the cell to prevent this from happening? I have so far used a copper, and a
graphite anode.
Also, if no material will prevent this from happening, I would like a question answered. If the cathode has a greater surface area than the anode,
will the net concentration of copper (II) acetate increase, or remain equal?
[Edited on 2-7-2012 by liquidlightning]
|
|
|
Diablo
Hazard to Others
  
Posts: 113
Registered: 17-9-2011
Member Is Offline
Mood: Autodidactic
|
|
Copper anode dissolves in acid to form copper acetate
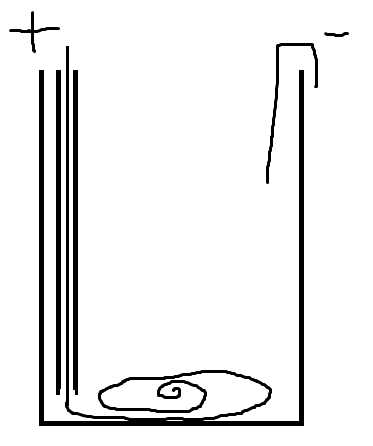
In a cell built this way little copper acetate will plate back out.
|
|
|
liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
But wouldn't most of the acid not be converted in a cell layout like that?
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
If you want full conversion and a pure product
https://www.youtube.com/watch?v=ieAvEXuneCw
Just dissolve the crystals and done. Pure copper acetate sol.
|
|
|
liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
I know about the hydrogen peroxide route, however that uses a lot of peroxide, is fairly expensive, and takes much longer to boil down/evaporate due
to the extra volume of the peroxide. Unless of course you use higher concentration peroxide, which is both more expensive, and not currently available
to me.
Thus, as electricity is cheap, I have opted for the electrochemical method.
|
|
|
liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
Oh, and I have assembled a cell as per Diablo's extraordinately detailed diagram, and am awaiting completion.
|
|
|
Diablo
Hazard to Others
  
Posts: 113
Registered: 17-9-2011
Member Is Offline
Mood: Autodidactic
|
|
I forgot to label the tube on the diagram. The cell works because the + wire is in a tube until it reaches the bottom of the cell where the copper
dissolves and the copper ions stay at the bottom of the solution allowing a lot of acetate to be formed before any copper ions reack the cathode.
|
|
|
liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
I realized that, although I used tape, not a tube. I have been pushing 65 watts through it with 200 ml of 10% acid for a few hours now, and a fair
amount of the acetate has gotten to the top electrode. I have been getting growths of copper crystals, but not nearly as large or fast as my previous
attempts with side by side electrodes. The water has gotten pretty warm so far. Just to be clear, the reaction is mostly finished when the positive
electrode starts to bubble hydrogen, correct?
Also, when I tried to make a salt bridge earlier, with a paper towel rolled up and draped into two beakers of the acid, for some reason it would not
work. It just wouldn't conduct electricity. Anyone know why?
[Edited on 3-7-2012 by liquidlightning]
|
|
|
Diablo
Hazard to Others
  
Posts: 113
Registered: 17-9-2011
Member Is Offline
Mood: Autodidactic
|
|
The + electrode will bubble if the acids used up, but its making oxygen not hydrogen. The paper salt bridges drastically reduce the current flow
through the cell, however if no current was flowing maybe it wasn't saturated with acid.
|
|
|
liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
Well, the copper acetate has diffused too much to the top, so I will distill off excess acid and concentrate the copper acetate, and run it again with
the distillate. Oh and yeah, my mistake, oxygen on the cathode.
[Edited on 3-7-2012 by liquidlightning]
|
|
|
roupt
Harmless

Posts: 3
Registered: 1-12-2007
Location: Lithuania
Member Is Offline
Mood: No Mood
|
|
Acetic acid route won't be efficient in electrolysis... try to make copper hydroxide and then neutralize. 12AX7 has covered this procedure on his site

|
|
|
liquidlightning
Hazard to Self
 
Posts: 66
Registered: 10-5-2012
Location: Washington
Member Is Offline
Mood: Witty
|
|
Why won't it be efficient?
|
|
|
roupt
Harmless

Posts: 3
Registered: 1-12-2007
Location: Lithuania
Member Is Offline
Mood: No Mood
|
|
Due to low acetic acid Ka value
|
|
|