| Pages:
1
2
3
4 |
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Your equations don’t balance.
I’ve never seen this synthesis before, but It doesn’t look too reliable. Ammonium + bleach in a neutral solution producing
NCl<sub>3</sub>? I just can’t picture that.
>The liquid is then placed in a plastic testube or beaker (NO GLASS!)
NCl<sub>3</sub> explodes violently on contact with any organics. These instructions look like BS to me.
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
Sorry, i meant to type "a glass testube or flask (NO PLASTIC!)". Although this reaction would most likely not cause an explosion should it have been
carried out in a plastic container. As the NCl3 is produced it is destroyed by the plastic so no amount can collect. I see the mistake in my equation,
it should be
3NaOCl + NH4Cl = NCl3 + 3NaOH + HCl
NCl3 + 3NaOH + HCl = NCl3 + 2NaOH + H2O +NaCl
And the solution is not neutral, it's acidic.
I will post pictures soon.
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|



As you can see, yellow droplets of NCl3 formed when I combined Ammonium Chloride and Sodium Hypochlorite in the precense of HCl.
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Interesting pictures, I don't think I've seen NCl3 before. What happened to it after you took the pictures? I believe it is supposed to decompose on
its own if it does not detonate first.
Hodges
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Yes, this synth of mbrown3391 DOES work! I've done it myself many years ago. As I have NH4Cl, I simply dissolved some NH4Cl in dilute HCl (it does not
dissolve well in conc. HCl) and then added NaClO (10%) drop by drop. I obtained a few drops of a yellow oil, with a very pungent, somewhat spicey
smell (once you've smelled it, you'll recognize it the rest of your life). These drops are very much like the drops shown in the pictures above, so I
think mbrown3391 did a good job making NCl3.
I just did the experiment and destroyed the stuff by slowly increasing the pH of the liquid. I was too scared to do real experiments with it  . At high pH it starts bubbling and disappears, I think it forms N2 and hypochlorite
at higher pH. . At high pH it starts bubbling and disappears, I think it forms N2 and hypochlorite
at higher pH.
Using NCl3 to make NI3 from KI seems like idiocy to me. If you really want to make NI3 from KI, then simply dissolve the KI in dilute HCl, and add
excess dilute H2O2. Iodine is formed and quickly settles at the bottom. Rinse well with water, no need to dry. To this, add concentrated NH3 and you
get your NI3.
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
After i took the pictures, i detonated it using the following setup. As you can see i only had a drop of it, so the explosion was not very big. It
made a cracking noise, like NI3.
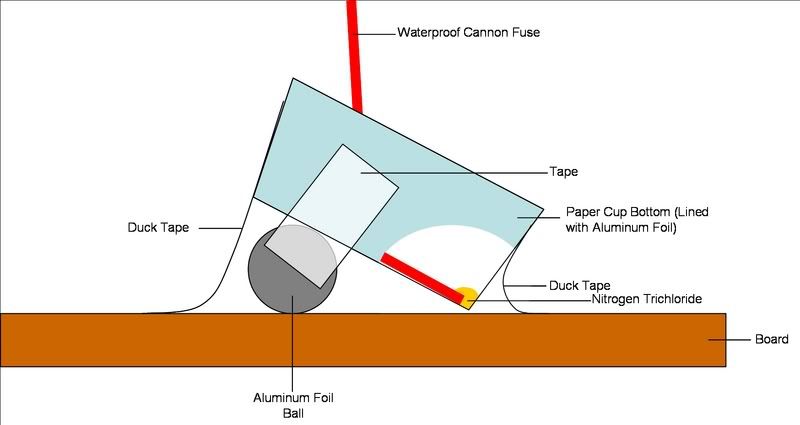
The explosion completely destroyed the cup, but did not damage the board.
Also i dont think the equation i posted previously is correct, as when i mixed the reactants i observed a lot of bubbling. Does anybody have any idea
what the correct equation would be?
[Edited on 10-9-2006 by mbrown3391]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I too did this once a year or so ago, just made a miniscule drop though. Will try it again as soon as I can find my soft plastic pipettes and dig up
the plastic glassware from an old chemistry set(what do you know, it DOES have a use).
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
On my first try, i immediately destroyed the NCl3 with NH4OH. This works pretty quickly. I am wondering how its discoverer lost three fingers and an
eye from this stuff. How much did he make and what made it explode?
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
Rougue Chemist, you cant make this stuff in plastic, i tried it. as the NCl3 settles to the bottom, it reacts with the plastic and becomes something
other than NCl3. I made this mistake the first time. Unfortunately glass is the only other option.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
The stuff detonates in contact with most anything. Even a fingerprint on the glass will cause it to detonate(supposedly). Back then chemists worked
with larger ammounts of chemicals, if you read the older preparatory books you will find all sorts of examples of quite dangerous chemicals being made
large scale. Like the preparation of 10g pure Mn2O7
EDIT: Really? reacts with plastic, hmm, how unfortunate.
[Edited on 10-9-2006 by rogue chemist]
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
So what is the largest amount you would prepare and detonate at one time?
EDIT: Yes. The first time i did it in a styrofoam cup (dont ask me why) and it ate right through the bottom. Then i tried it in a soda bottle. This
time it didn't eat through but no drops formed, it just turned the bottom of the bottle yellow.
[Edited on 10-9-2006 by mbrown3391]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Well pretty much anything organic eats through polystyrene, hope I don't have to break out the teflon here.
As for how much one should make, that is up to you, taking into consideration how much damage you could do to yourself in the worst case scenario. In
my book I probably would not try to go above 0.2 ml. After all, this is a novelty, not for practical use.
Even the old preparatory books don't make NCl3 pure, they make and keep it dissolved in carbon tetrachloride. When they take such precautions, you
know it is serious stuff.
[Edited on 10-9-2006 by rogue chemist]
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
In my electrolysis experiment a while back I used a plastic container. That may explain the lack of any yield.
Hodges
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
Thats was definately the problem. Common sense tells you to use plastic so you dont get shrapnel if there was an accident, but in this case you have
to use glass.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I always assumed that you needed Cl<sub>2</sub> to make NCl<sub>3</sub>. In woelen’s procedure, this is made in situ from
the acid + chloride + hypochlorite.
I don’t see where the chlorine comes from in your procedure, though. Maybe it isn’t necessary after all?
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
I think this is actually the correct equation:
3NaOCl + NH4Cl + HCl = NCl3 + Cl2 + 3NaOH + H2O
Much of the free chlorine produced escapes but some will react in the following manner:
Cl2 + NaOH = NaOCl + HCl
Cl2 + H2O =HClO + HCl
2HCl + 2NaOH = 2NaCl + 2H2O
As you can see, the reaction ultimately produces NaOCl and HOCl along with the initial NCl3. Both would react with the remaining ammonium chloride to
produce more NCl3. After one round of this cycle, the products are:
NCl3
NaOCl
HOCl
2NaCl
3H2O
This appears to allow the reaction to last much longer than it normally would. This is good for high yeild, but in a labratory experiment it is
dangerous, making it difficult to judge how much sodium hypochlorite you should add initialy.
[Edited on 11-9-2006 by mbrown3391]
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
im trying to determine some exact ammounts here, but i dont know the percentage of NH4OH in Blue Ribbon Ammonia. Does anyone have any idea?
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
It seems that household ammonia solutions tend to be 2M to 3M concentration. I recall titrating some grocery store clear ammonia solution once and
finding it was just over 2M.
Hodges
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I'm not sure where your equations come from. It's more like this:
First chlorine is formed in the acidic environment. Note that commercial bleach already contains more than enough chloride ions.
2H<sup>+</sup> + Cl<sup>-</sup> + OCl<sup>-</sup> --> Cl<sub>2</sub> + H<sub>2</sub>O
This is the step I’m not positive about, but it looks about right:
3 Cl<sub>2</sub> + NH<sub>4</sub><sup>+</sup> --> NCl<sub>3</sub> + 4H<sup>+</sup> +
3Cl<sup>-</sup>
Overall,
3OCl<sup>-</sup> + NH<sub>4</sub><sup>+</sup> + 2H<sup>+</sup> --> 3H<sub>2</sub>O +
NCl<sub>3</sub>
That's why I question a neutral synthesis.
[Edited on 11-9-2006 by neutrino]
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
I know that a large amount of free chlorine is formed from this synthesis that is never part of the creation of NCl3, because it goes into the air.
| Quote: |
3OCl- + NH4+ + 2H+ --> 3H2O + NCl3 |
Your eqution does not include this.
EDIT: Unless maybe all the NCl3 is made in the few seconds it takes for the chlorine gas to rise out of the liquid? If this is the case, then yeild is
severely lowered. Maybe adding bleach very slowly with a pipette into a flask stopped with a one-hole stopper would help. This way almost all of the
chlorine could be absorbed.
[Edited on 12-9-2006 by mbrown3391]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Neutrino's equation is a net equation, and it seems correct to me. The intermediate is chlorine gas, but not all of it reacts. But that does not
invalidate the net equation given. If a closed system were used, with excess NH4Cl and acid, then all hypochlorite would be used up in formation of
NCl3.
There are, however, side reactions, such as formation of nitrogen gas, and possibly some hydrazine as well.
|
|
|
2bob
Harmless

Posts: 21
Registered: 29-8-2006
Member Is Offline
Mood: No Mood
|
|
is it possible to make NaNO2 from ammonia?
I was thinking along the lines that if H2O2 + NH4OH = NH3NO2 + H20, then wouldn't NaOCO2 (Sodium percarbonate: in all the new bleaches) which breaks
down to H202 + NaCO2 (in aq soln), break the bond between the NH3 & NO2 giving NaNO2 + NH3CO2 (which would precipitate, wouldn't it?) leaving pure
NaNO2 in aqueous solution?
I am unsure of the chemistry here, however it appears possible from my somewhat limited experience, and possibly overly imaginative perspective?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
NH4NO2 = N2 + 2H2O. (Oh, and subtle difference: it's aich-two-oh (H2O), not aich-twenty.  ) )
What is NH3NO2, NaOCO2 and NH3CO2?
I'm sure you meant NH4, Na2CO3.H2O2 (I forget how much H2O2) and (NH4)2CO3.
There is a true sodium percarbonate NaCO3 or so, and similarly perborate as well, but they are NOT the common adduct sold as "oxiclean", et al.
Ammonium carbonate is soluble; sodium bicarbonate is probably one of the least soluble alkali carbonates.
To oxidize something, you have to go through the intermediate states. You might get something like NH3 + 1.5 H2O2 = 3H2O + N, where N is a nitrogen
radical or so (N(0), which readily combines with others to form diatomic nitrogen N2). The radical may combine with more oxidizer, for instance N +
2H2O2 = 2H2O + NO2, which disproportionates in solution 2NO2 + H2O = NO3- + NO2- + 2H+, which oxidizes further as NO2- is sensitive. But I doubt this
reaction is particularly common in solution.
More often, ammonia is burned with oxygen on a hot catalyst, where such reactions are encouraged by the freedom of thermal motion and the action of
the catalyst. The process is something like 2NH3 + 5O = 2NO + 3H2O, where NO is formed because NO2 disproportionates at this temperature (NO + O
<--> NO2, which proceeds to the right at lower temperatures).
Tim
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Forget about making NaNO2 from ammonia in a simple aqueous redox reaction. If ammonia is oxidized in aqueous solution (and it isn't by the
peroxo/carbonate adduct you mention), then usually N2 is formed, sometimes hydrazine or hydroxylamine.
NaCO2 is something non-existent. You probably mean Na2CO3 (sodium carbonate). The sodium percarbonate you mention is a compound, which can be written
as Na2CO3.xH2O2.yH2O (I don't know the precise values of x and y). When this is dissolved, you get free H2O2, sodium carbonate and water.
|
|
|
2bob
Harmless

Posts: 21
Registered: 29-8-2006
Member Is Offline
Mood: No Mood
|
|
ta.
|
|
|
| Pages:
1
2
3
4 |