| Pages:
1
2 |
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Toxic-gas liberating explosives
Here are a few ideas for energetic compounds that would liberate toxic gasses when detonated:
(CH2OH)2 + 2HCl --(H2SO4)--> (CH2Cl)2 + 2H2O
(CH2Cl)2 + 2HNO3 --(H2SO4)--> (CHClNO2)2 + 2H2O
(CHClNO2)2 + 2Cl2 --> (CCl2NO2)2 + 2HCl
3(CCl2NO2)2 + 2Na3P --> (CClNO2)6P2 + 6NaCl
(CClNO2)6P2 --> 2CO + CO2 + 3N2 + P2O5 + 3COCl2
CH2Cl2 + HNO3 --(H2SO4)--> CHCl2NO2 + H2O
2CHCl2NO2 --> N2 + CO2 + COCl2 + H2O
CH3CH2OH + 3Cl2 --> CCl3CH2OH + 3HCl
CCl3CH2OH + HNO3 --(H2SO4)--> CCl3CH2NO3 + H2O
CCl3CH2NO3 --> 3COCl2 + CO + 2H2O + N2
CH3COOH + 3Cl2 --> CCl3COOH + 3HCl
CCl3COOH + CH3CH2OH --(H2SO4)--> CCl3COOCH2CH3 + H2O
CCl3OOCH2CH3 + NH3 --> CCl3ONH2 + CH3CH2OH
CCl3CONH2 + HNO3 --(H2SO4)--> CCl3CONHNO2 + H2O
2CCl3CONHNO2 --> 3COCl2 + CO2 + 2N2 + H2O
(CH2OH)2 + HF --(H2SO4)--> (CH2OH)(CH2F) + H2O
(CH2OH)(CH2F) + 4Cl2 --> (CCl2OH)(CCl2F) + 4HCl
(CCl2OH)(CCl2F) + CH3CH2OH --(H2SO4)--> (CCl2OCH2CH3)(CCl2F) + H2O
(CCl2OCH2CH3)(CCl2F) + NH3 --> (CCl2NH2)(CCl2F) + CH3CH2OH
(CCl2NH2)(CCl2F) + HNO3 --(H2SO4)--> (CCl2NHNO2)(CCl2F) + H2O
(CCl2NHNO2)(CCl2F) --> 2COCl2 + HF + N2
3CH3CHO + 3H2O2 --(H2SO4)--> (CH3CHOO)3 + 3H2O
(CH3CHOO)3 + 12Cl2 --> (CCl3CClOO)3 + 12HCl
(CCl3CClOO)3 --> 6COCl2
CH3CHOHCH2CH3 + HClO4 --> CH3CHClO4CH2CH3 + H2O
CH3CHClO4CH2CH3 + 9Cl2 --> (CCl3)(CCl)(ClO4)(CCl2)(CCl3) + 9HCl
(CCl3)(CCl)(ClO4)(CCl2)(CCl3) --> 4COCl2 + Cl2
(CH2OH)2 + 2HCl --> (CH2Cl)2 + 2H2O
(CH2Cl)2 + HNO3 --(H2SO4)--> (CHNO2Cl)(CH2Cl) + 2H2O
2(CHNO2Cl)(CH2Cl) --> 4CO + 4HCl + H2 + N2
CH3CH2CH2OH + 3Cl2 --> CCl3CH2CH2OH + 3HCl
CCl3CH2CH2OH + HNO3 --(H2SO4)--> CCl3CH2CH2NO3 + H2O
2CCl3CH2CH2NO3 --> 6CO + 6HCl + N2 + H2
CH3COOH + 3F2 --> CF3COOH + 3HF
CF3OOH + CH3CH2OH --(H2SO4)--> CF3COOCH2CH3 + H2O
CF3COOCH2CH3 + HNO3 --(H2SO4)--> CF3COOCH2CH2NO2 + H2O
2CF3COOCH2CH2NO2 --> 8CO + 6HF + N2 + H2
CH3COOH + 3Cl2 --> CCl3COOH + 3HCl
CCl3COOH + CH3CH2OH --(H2SO4)--> CCl3COOCH2CH3 + H2O
CCl3COOCH2CH3 + HNO3 --(H2SO4)--> CCl3COOCH2CH2NO2 + H2O
2CCl3COOCH2CH2NO2 --> 8CO + 6HCl + N2 + H2
I weep at the sight of flaming acetic anhydride.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Nitrotrichloroacetamide (CCl3CONHNO2) would be a very effective chemical weapon. It is an explosive compound, that, when detonated, would release
large quantities of phosgene (COCl2) gas. Approximately 716g of COCl2 would be released when 1000g of CCl3CONHNO2 is detonated. It could be easily
prepared by one equipped with a twisted mind. One kilogram of CCl3CONHNO2 would produce enough phosgene to cause ~2148000L of air to have an
effectively lethal concentration of phosgene.
Synthesis of CCl3COOH
Acetic acid is reacted with chlorine gas in the presence of ultraviolet light.
Place 60g (58mL) of 100% CH3COOH in a 100mL flask. Set up a "blacklight" (ultraviolet-light emitting lightbulb) directly adjacent to the flask, and
turn it on. Keep the temperature of the contents of the flask around 20C. Proceed to gently jet chlorine gas into the flask until the mass of the
contents of the flask has reached about 163.5g. Be sure to either use a gas mask, or to be well clear of this reaction; dangerous amounts of HCl gas
is produced; and obviously, working with chlorine gas is hazardous.
CH3COOH + 3Cl2 --(UV)--> CCl3COOH + 3HCl
Synthesis of CCl3COOCH2CH3
Trichloroacetic acid (CCl3COOH) is reacted with an excess of ethanol in the presence of concentrated sulfuric acid.
Place 163.5g of CCl3COOH in a 500mL flask. Add 60g CH3CH2OH (76mL) to the beaker. Add 10mL of concentrated sulfuric acid. Allow the contents of the
flask to react for several days, keeping the flask stoppered. After the reaction has completed, pour the contents of the flask into an evaporating
dish, and allow it to evaporate until all of the water and ethanol has evaporated. Place the CCl3COOCH2CH3 in a 600mL beaker, and carefully rinse it
with water for several minutes (this removes the remaining sulfuric acid).
CCl3COOH + CH3CH2OH --(H2SO4)--> CCl3COOCH2CH3 + H2O
Synthesis of CCl3CONH2
Ethyl trichloroacetate (CCl3COOCH2CH3) is reacted with aqueous ammonia at room temperature. The solution is then allowed to evaporate; CCl3CONH2 is
the remaining solid.
Place 191.5g of CCl3COOCH2CH3 in a 1000mL beaker. Slowly add ammonia solution until no more CCl3CONH2 is precipitating upon further addition of
ammonia solution. Allow the contents of the beaker to evaporate. The resulting solid is CCl3CONH2.
CCl3COOCH2CH3 + NH3 --> CCl3CONH2 + CH3CH2OH
Synthesis of CCl3CONHNO2
Trichloroacetamide is nitrated with mixed acid; the mixed acid is kept cooled in an ice bath. Temperatures are kept below 10C.
Prepare a mixture of 63g HNO3 (42mL) and 98g H2SO4 (55mL) by slowly adding the HNO3 to the H2SO4 (which is in a 1000mL beaker; the beaker is in an ice
bath). Keep the temperature of the mixed acid below 10C. Then, *slowly* add 140g of CCl3CONH2 to the mixed acid, keeping the temperature below 10C at
all times. Allow nitration to proceed for several hours, monitored at all times. Then, dump the contents of the beaker into a glass fiber filter.
Rinse the solid that is filtered with 500mL of very cold water, to remove remaining sulfuric and nitric acid.
CCl3CONH2 + HNO3 --(H2SO4)--> CCl3CONHNO2 + H2O
Detonation of CCl3CONHNO2
2CCl3CONHNO2 --> 3COCl2 + CO2 + 2N2 + H2O
Predicted properties of CCl3CONHNO2:
Solid, insensitive explosive.
Properties of precursors:
CCl3COOH
Specific gravity: 1.62
Melting point: 58C
Boiling point: 196C
Very soluble in water. Soluble in alcohol and ether.
Appearance: crystalline solid.
CCl3COOCH2CH3
Specific gravity: 1.38
Melting point: unknown
Boiling point: 168C
Insoluble in water. Infinitely miscible in alcohol and ether.
CCl3CONH2
Specific gravity: unknown
Melting point: 141C
Boiling point: 239C
Very slightly soluble in water. Very soluble in alcohol and ether.
I weep at the sight of flaming acetic anhydride.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Here are a few energetic compositions that would release toxic gasses, and would be very simple to prepare:
5CH2Cl2 + 2NaNO3 --> Na2O + N2 + 5CO + 10HCl
5CH2Cl2 + 4NaNO3 --> 2Na2O + 2N2 + 5H2O + 5COCl2
10CH2Cl2 + 12NaNO3 --> 6Na2O + 6N2 + 20HCl + 10CO2 + 5O2
I weep at the sight of flaming acetic anhydride.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Sorry to blow your theory and to send all those reactions down the drain, but phosgene is thermodynamically unstable and decomposes above 300C....
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Exactly how does it decompose (equation please)? 
I weep at the sight of flaming acetic anhydride.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Whaddaya think?
COCl2 -> CO + Cl2 duh......
Tsss, all those equations above and you can't figure out this one?
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I have no idea why I asked that question. *smacks forehead* 
I weep at the sight of flaming acetic anhydride.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
How about a binary of H2 and F2 gas? They still react explosively at -200C  to
form HF, an extremely toxic gas. to
form HF, an extremely toxic gas.
Only problem is where to get the fluorine gas.
|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
hehe, even better, an explosive that liberates H2 and F2 so they too explode and then form HF. pretty sick
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Sorry Madscientist, but many of the equations you have given are right theorically but practically they are unfeasible or wrong!
5CH2Cl2 + 2NaNO3-->Na2O + N2 + 5CO + 10HCl
5CH2Cl2 + 4NaNO3-->2Na2O + 2N2 + 5H2O + 5COCl2
10CH2Cl2 + 12NaNO3-->6Na2O + 6N2 + 20HCl + 10CO2 + 5O2
Are absolutely wrong! CH2Cl2 boils at 40°C and the volatilisation cools down the reaction very fast; also CH2Cl2 is not very flamable, it only reacts
violently with Al ultrafine powder, solid Na or K!
Also:
2HCl + 1/2O2 --> H2O + 1/2Cl2
Na2O + 2HCl --> 2NaCl + H2O
Na2O + CO2 --> Na2CO3
Na2O + Cl2C=O --> 2NaCl + CO2
CCl3-CO-NH-NO2 should be very unstable -maybe so unstable that it doesn't exists; because CCl3 is first a very electroatractive group (thus CCl3-CO-
is rather acidic); a good leaving group too, nitramides are much less stable than nitramines; here you have a molecule that should be as (if not more)
unstable than dinitrourea.
CCl3-CO-OCH2-CH3 + NH3(dry) -solvant-> CCl3-CO-NH2 + CH3-CH2-OH
But
CCl3-CO-OCH2-CH3 + NH4OH --> CCl3-CO2NH4 + CH3-CH2OH
Finally:
(CH2OH)2 + 2HCl --(H2SO4)--> (CH2Cl)2 + 2H2O
You forgot:
+(CH2O)2SO2 + (CH2)2O + HO-CH2-CH2-Cl
(CH2Cl)2 + 2HNO3 --(H2SO4)-->(CHClNO2)2 + 2H2O
Unfortunately no; aliphatic nitrocompounds aren't made the same way as alcenic/benzenic nitrocompounds!
(CHClNO2)2 + 2Cl2 -->(CCl2NO2)2 + 2HCl
The first one is already very dangerous and unstable see related compounds like CH2Cl-NO2 and CHCl2-NO2!
3(CCl2NO2)2 + 2Na3P --> (CClNO2)6P2 + 6NaCl
Why not, possible, no fuking ID!
(CClNO2)6P2 --> 2CO + CO2 + 3N2 + P2O5 + 3COCl2
Might be P2O3, N2, CO2, CO and Cl2!
CH2Cl2 + HNO3 --(H2SO4)--> CHCl2NO2 + H2O
Maybe at under pressure boiling at reflux (with very big risks of explosion (CH2Cl-NO2 and CHCl2-NO2).
2CHCl2NO2 --> N2 + CO2 + COCl2 + H2O
Nothing new under the sun; it is known for ages that halonitrocarbons like Trinitrotrichlorobenzene, chloropicrine (CCl3-NO2-toxic on its own),
haloalkanes mixed with oxydisers like peroxydes, NH4NO3, N2O4, O3, liq O2; H2O2, HNO3 conc, HClO4 conc, ... do produce some free halide, CO and traces
of X2C=O + H2O and HX!
CH3CH2OH + 3Cl2 --> CCl3CH2OH + 3HCl
I'm almost sure that will never go this way, but more this way:
CH3-CH2OH + Cl2 --> CH3-CH=O + 2HCl
CH3-CH=O + Cl2 --> CH2Cl-CH=O + HCl
CH3-CH=O <--> CH2=CH-OH
CH2=CH-OH + Cl2 --> CH2Cl-CHClOH
CH2Cl-CHClOH --> CHCl=CHCl + H2O
CH2Cl-CHClOH --> CHCl=CHOH <--> CH2Cl-CH=O
...--> CHCl2-CHCl2 and CCl3-CH=O (chloral)
The action of NaOCl (Javel water) on ethanol provides partially chloral hydrate.
CCl3CH2OH + HNO3 --(H2SO4)--> CCl3CH2NO3 + H2O
CCl3-CH2O- must be quite acidic and thus the ester will not be stable, maybe doesn't exist!
CCl3CH2NO3 --> 3COCl2 + CO + 2H2O + N2
CH3COOH + 3Cl2 --> CCl3COOH + 3HCl
This one is true 
(CH2OH)2 + HF --(H2SO4)--> (CH2OH)(CH2F) + H2O
And also (CH2F)2!
(CH2OH)(CH2F) + 4Cl2 --> (CCl2OH)(CCl2F) +4HCl
See the reaction of ethanol with Cl2 and extrapolate to this twin brother!
(CCl2OH)(CCl2F) + CH3CH2OH --(H2SO4)--> (CCl2OCH2CH3)(CCl2F) + H2O
Why not!
(CCl2OCH2CH3)(CCl2F) + NH3 --> (CCl2NH2)(CCl2F) + CH3CH2OH
Maybe, maybe not; possible reaction of the halide with the amine...cf CH3-NH2 with CH3-Cl
--> CH3-NH2Cl-CH3 --> (CH3)3NHCl --> (CH3)4NCl
(CCl2NH2)(CCl2F) + HNO3 --(H2SO4)--> (CCl2NHNO2)(CCl2F) + H2O
Primary amine doesn't form nitramines in presence of H2SO4...do you do RDX with H2SO4/HNO3 or with HNO3 conc with other dehydratants than H2SO4? True
that H2SO4 is used in NITRAMIDES synthesis not in nitraMINes!
(CCl2NHNO2)(CCl2F) --> 2COCl2 + HF + N2
3CH3CHO + 3H2O2 --(H2SO4)--> (CH3CHOO)3 + 3H2O
I like the idea, but nowhere I have seen any mention of acetaldehyde peroxyde; nor of formol peroxyde!This must mean they are so unstable they
decompose immediately at ambiant T or while forming!
(CH3CHOO)3 + 12Cl2 --> (CCl3CClOO)3 + 12HCl
It would be more like: --> HCCl3 + H2O + HCl + CO2 + CCl3-CH=O + CH3-CO2H + CCl3-CO2H
(CCl3CClOO)3 --> 6COCl2
Noway; but CH3-CO-CH3 --> ClCH2-CO-CH3 and ClCH2-CO-CH2Cl that may form a CTAP brother halogenated that will free traces of phosgene
Those halogenated CTAP will have higher densities, better oxygen balance (since Cl2 is an oxydiser) and thus display higher VODs!
Haloacetons are very reactive compounds they can polymerise explosively on their own, they are strong lacrymator and must be suffocating gases like
any halogen reactive containing compound (Cl-CN, Cl2, Cl2C=O, Cl2O, ClO2, Cl2S, TiCl4, AlCl3, NCl3, ....).
CH3CHOHCH2CH3 + HClO4 --> CH3CHClO4CH2CH3 + H2O
True but perchloric ester are spontaneously detonating compounds at ambiant T!
CH3CHClO4CH2CH3 + 9Cl2 --> (CCl3)(CCl)(ClO4)(CCl2)(CCl3) + 9HCl
Detonation will occure before the molecule has any chance to react that far!
(CCl3)(CCl)(ClO4)(CCl2)(CCl3) --> 4COCl2 + Cl2
No comments!
(CH2OH)2 + 2HCl --> (CH2Cl)2 + 2H2O
True!
(CH2Cl)2 + HNO3 --(H2SO4)--> (CHNO2Cl)(CH2Cl) + 2H2O
Only under vapour phase at high T (400°C) no need of H2SO4!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
2(CHNO2Cl)(CH2Cl) --> 4CO + 4HCl + H2 + N2
Yep!
CH3CH2CH2OH + 3Cl2 --> CCl3CH2CH2OH + 3HCl
Not accesible that way!
CCl3CH2CH2OH + HNO3 --(H2SO4)--> CCl3CH2CH2NO3 + H2O
Interesting high density, for sure!
2CCl3CH2CH2NO3 --> 6CO + 6HCl + N2 + H2
Yep!
CH3COOH + 3F2 --> CF3COOH + 3HF
F2 often explodes upon contact with organics!
CF4 + CO2 + HF are the results!
CF3COOH + CH3CH2OH --(H2SO4)--> CF3COOCH2CH3 + H2O
Yep!
CF3COOCH2CH3 + HNO3 --(H2SO4)--> CF3COOCH2CH2NO2 + H2O
Nope esters doesn't survive acids!And alkyl nitrocompounds does only form at high T (350-450°C)!
You will more likely form CH3-CH2-ONO2 and CH3-CH2-SO4H with serious risks of explosion owing to the very strong acidic trifluoroacetic acid!
2CF3COOCH2CH2NO2 --> 8CO + 6HF + N2 + H2
Yep, but made via nitroethanol (formol/nitromethane 1/1-10).
CCl3COOCH2CH3 + HNO3 --(H2SO4)--> CCl3COOCH2CH2NO2 + H2O
Same as above!
2CCl3COOCH2CH2NO2 --> 8CO + 6HCl + N2 + H2
Yep!
PH Z
|
|
|
TheBear
Hazard to Self
 
Posts: 78
Registered: 17-10-2002
Location: Sweden
Member Is Offline
Mood: distilled
|
|
Doesn't
NH4N3 liberate toxic gases. This might be wrong, I'm a bit tired at the moment but I recall reading about it at E&W forum. However it's currently
down.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Nope, just N2 and H2....
If it wouldn't produce N2, it wouldn't be explosive at all, because the triple bond formation releases so much energy.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Wouldn't ammonia be one of the products of the detonation of ammonium azide? Ammonia has an exothermic heat of formation, if I remember correctly,
which would mean that it wouldn't break down into its elements.
I weep at the sight of flaming acetic anhydride.
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
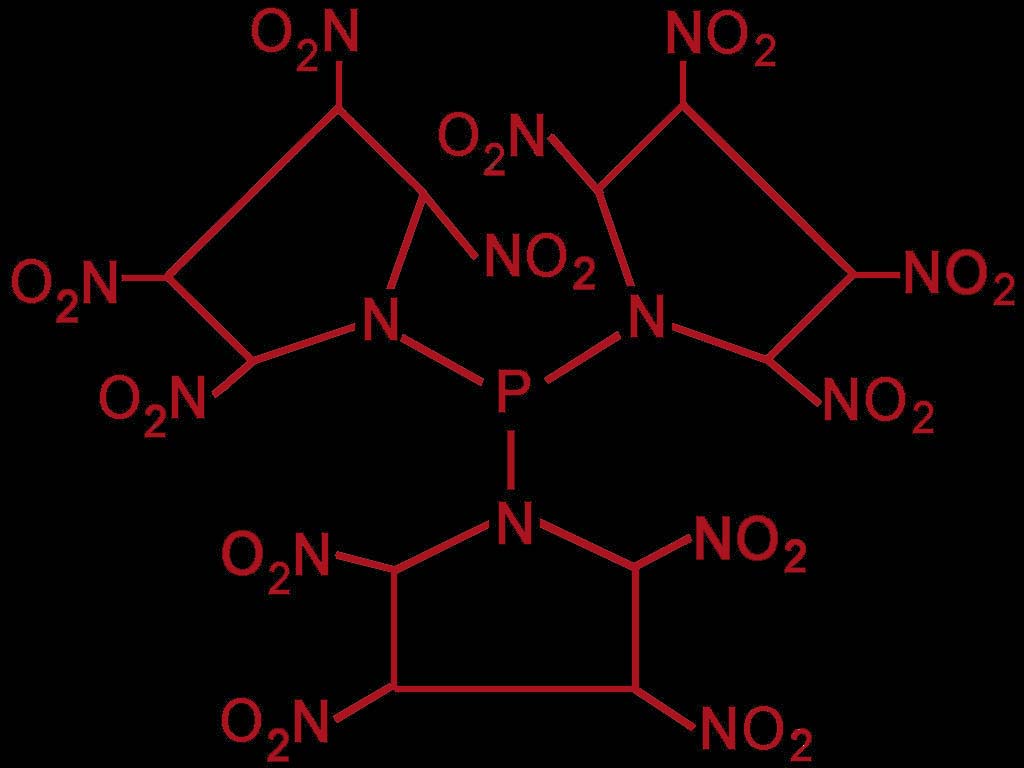
213 new ideas
I think it`s possible making high nitrated explosives, when bounding phosphorus with other chemicals, for instance
tri(2,3,4,5-tetranitro-1-tetramethyleneiminyl)phosphine.
[TTTIPP]
This explosive must be protected from
agressive chemicals or it will decompose !!!
I post the chemical structure later.
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
chemical structure
Here the chemical structure of the pyrrolidine-based component.
Does anybody have ideas for a easy synthesis-way to make this explosive ?
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
Sorry, the picture was not submited in my last post !
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
ok, I'm not too good (I actually suck) with these chemical equation things but NI3 does give out iodine gas and iodine is poiosonous
|
|
|
Haggis
Hazard to Others
  
Posts: 238
Registered: 1-12-2002
Location: Mid-America.
Member Is Offline
Mood: Lacrymating
|
|
Yes, Iodine vapor wouldn't be good for you, but the purpose of having an explosive that liberates toxic gas is to actually be able to use the
explosive. NI3 would just be too sensitive to have any practicality other than entertaining chemistry students. Wouldn't the equation go like
this?
2NI3--->3I2+N2 Wouldn't every 1069.4 grams of NI3 decompose to 761.4 grams of I2? No person is insane enough to deal with a kilogram of
NI3, the amount of I2 produced at 5 grams is quite high, 3.56 grams. Although it gives off a lot of I2 per gram of NI3, the explosive part of it is
the problem.
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
how did Mega set it off? He has some pictures and I tihnk a video of him setting off large pile of it with a feather... isn't that kinda
dangerous? someone can set it off like he did
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
http://chemed.chem.purdue.edu/demos/main_pages/5.5.html
this may be not what you're exactly looking for but at least close
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Krypton,
Do you have any reference for the building bloc of your idea?
cyclo(-NH-CHNO2-CHNO2-CHNO2-CHNO2-)
Should be hell of a good explosive on its own...especially the nitramine... perfect OB 
O2N-N(CHNO2-CHNO2)2 --> 4CO2 + 2H2O + 5/2N2
High density but must be sensitive!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Cappy
Hazard to Self
 
Posts: 92
Registered: 27-3-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by madscientist
Nitrotrichloroacetamide (CCl3CONHNO2) would be a very effective chemical weapon. It is an explosive compound, that, when detonated, would release
large quantities of phosgene (COCl2) gas. Approximately 716g of COCl2 would be released when 1000g of CCl3CONHNO2 is detonated. It could be easily
prepared by one equipped with a twisted mind. One kilogram of CCl3CONHNO2 would produce enough phosgene to cause ~2148000L of air to have an
effectively lethal concentration of phosgene.
|
Holy shit, that would be a lethal radius of 12.9 m or over 42 ft. If you wer considering a hemisphere instead of a sphere, that would be a radius of
16.3 m or over 53 ft. 
I sincerely hope all of this is hypothetical. 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
716g of Cl2C=O is 7,24 moles
A 100% cloud of phosgene would fill a volume at STP of 162,24 liters but here we are in a detonation process where the heat is much higher (let say
1500°C); volume reaches 1160 liters then under atmospheric pressure.
Of course 100% phosgen is lethal but 1% is lethal too; it is only a mather of time exposure!If toxicity range is known you may know the limit range of
lethal action!
From the datas of madscientist: it seems
toxicity is at 0,0075% by volume!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
"Nitrogen trichloride, also called nitrogen chloride, agene, chlorine nitride, trichloramine, trichlorine nitride, chloride of azode, or
Stickstofftrichlorid, is an unstable primary explosive compound. Its preparation is not complicated and the chemicals used are simple, cheap, and
readily obtainable. You could pump the stuff out by the liter if it was not so sensitive. Nitrogen trichloride will explode if heated, exposed to
sunlight, or mixed with organic compounds. It does not like to be friendly around many other chemicals, shock, sparks, and it will explode if frozen
and thawed. The explosive properties were first reported in the 18th century by Sir H. Davy, he had this to say: "The fulminating oil which you
mentioned roused my curiosity and nearly deprived me of an eye. After some months of confinement I am again well." Ouch, that must have
hurt."
Sounds interesting?
Nitrogen trichloride decomposes like this: 2NCl3->N2 + 3Cl2.
That's how it's made:
"Dissolve 30 g of ammonium nitrate in 70 mL water in a 200-mL Erlenmeyer flask. Prepare a chlorine generator as described in the synthesis
section. Place a tube connected to the generator at the bottom of the flask so the chlorine gas can bubble into the liquid, a bubbler will help a lot
with the reaction. Gently heat the flask to start the reaction while adding chlorine gas. An oily yellow liquid will begin to appear on the bottom of
the flask, that is the nitrogen trichloride. Stop heating the flask when the drops appear. After 20 to 30 minutes the reaction should be complete. Use
a medicine dropper to extract the nitrogen trichloride from the flask, transfer it to a small test tube and remove any water accidently sucked up with
it. You will need a graduated cylinder for measuring liquids. This explosive will decompose within 24 hours of its preparation."
|
|
|
| Pages:
1
2 |