ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Is it an enolate?
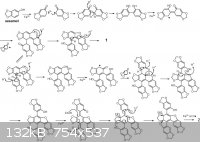
I want to know if the product from the Fe3+ catalized oxidation of sesamol is an enolate. Is it an enolate? It's reacting with itself. Is it an aldol
condensation?
http://www.sciencedirect.com/science/article/pii/S0040403909...
"Imagination is more important than knowledge" ~Einstein
|
|
|
triplepoint
Hazard to Others
  
Posts: 127
Registered: 11-4-2012
Location: U.S.
Member Is Offline
Mood: in equilibrium
|
|
I believe not. I think that an enolate requires a double bond between O and C. I believe that an aldol condensation also requires that you begin
with a double bond between O and C.
I am sure that you will receive more detailed answers form those more knowledgeable than I.
|
|
|
Siggebo
Harmless

Posts: 23
Registered: 6-5-2012
Location: Sweden
Member Is Offline
Mood: Under reflux
|
|
The first reaction step, you mean?
The Ar-OH ring you have there is actually an enol (ph-enol, eh? ) and it's
equilibrium is far to the enol side. The first step seems to be a conversion into the keto tautomer (with Fe3+ as acid catalyst) and then you have a
radical reaction, not an aldol-type reaction. You can tell by the "fish-hook" arrows, with one hook denoting one electron. Compare the two-hook
ordinary curly arrows indicating electron pair movement. ) and it's
equilibrium is far to the enol side. The first step seems to be a conversion into the keto tautomer (with Fe3+ as acid catalyst) and then you have a
radical reaction, not an aldol-type reaction. You can tell by the "fish-hook" arrows, with one hook denoting one electron. Compare the two-hook
ordinary curly arrows indicating electron pair movement.
And do correct me if I'm wrong. It happens all the time.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
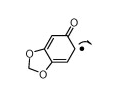
I was trying to ask if this particular molecule was an enol. It has no name. Let's call it 1-ketyl-3,4-Methylenedioxybenzene.
"Imagination is more important than knowledge" ~Einstein
|
|
|
Siggebo
Harmless

Posts: 23
Registered: 6-5-2012
Location: Sweden
Member Is Offline
Mood: Under reflux
|
|
No, it is not. Enolates have a negative oxygen next to a C-C double bond.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
You're right. I missed that small detail. Thanks for the answers!
 Cool! Cool!
"Imagination is more important than knowledge" ~Einstein
|
|
|