elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Preparation of Tetralin?
First post in the Organic section. I'm quite afraid for my life. XD
Anyway, I was wondering how I would go about making tetrahydronapthalene (aka tetralin) from mothball-grade napthalene. I understand sodium, ethanol
and possibly molten naptha are involved, but the description was a little odd.
Quote: "There is also a published synth using sodium in ethanol to reduce naphthalene to tetralin and then on to decalin. I've actually run this
reaction, and yields are a lot higher using a neutral (decane) hydrocarbon with naphthalene dissolved in it, adding sodium, and reducing with
ethanol."
Src: http://www.instructables.com/id/Make-Potassium-Metal/
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Dave Angel
Hazard to Others
  
Posts: 128
Registered: 22-3-2005
Location: UK
Member Is Offline
Mood: 0 K
|
|
I found a prep as part of a multi-step synthesis on Organic Syntheses:
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv6p0731
The caveat is that it does involve sodium and liquid ammonia - so you'd have to be familiar with the prep and handling of that.
As for less agressive routes, nothing is immediately evident to me, but then (as one might expect) hydrodgenation of napthalene does not appear to be
a gentle process in general...
A nice suggestion for napthalene all the same; I see myself adding it to my ever-growing project list, if only to find something to do with my
mothballs that have been hanging around unproductively for about 10 years!
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
The metal-ammonia type (Birch reduction) is good to hydrogenize aromatic rings, the only problem that it's not selective, it's smelly, the amides what
got are quite explosive when they contact with water and it is really expensive.
There is an old method for the selective hydrogenization of one ring from the naphthalene. Nitrate it, make mononitronaphtalene, reduce it with any
method (Fe + HCl ect) and then with PtO2 (as I remember correctly) and just that ring will get saturated what contains the amino group). And the
diazotate it and remive the amine somehow (several methods are known).
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Nitrate it with nitric acid or a salt?
How do I tell when it's reduced?
Where on earth would I get platinum oxide?
Diazotate?
I was thinking I could do it with ethanol and sodium, why would this work (or additionally, why wouldn't it)?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Dave Angel
Hazard to Others
  
Posts: 128
Registered: 22-3-2005
Location: UK
Member Is Offline
Mood: 0 K
|
|
Out of interest, is tetralin your specific goal or are you just looking for a reaction to do with the napthalene?
For decenes and decane, the Benkeser reaction may be of interest, if you can obtain/prepare suitable amines - your lithium coming from batteries.
Rhodium also has some interesting notes on Birch-like reductions.
I'm sure kristofvagyok will come back on your specific questions, but in the mean time you may be interested to read into diazonium compounds, these being useful intermediates indeed, the key reagent to obtain being sodium nitrite
(NaNO2)
Also, one could buy PtO2 (assuming you have access to a suitable supplier), or make yourself by first forming the nitrate then calcining.
All of this may not be necessary though - essentially, it all comes back to whether you want tetralin to be your end product or not?
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
I think Na/ethanol gives the dihydro cpd., and you'd want boiling pentanol instead.
The hydrogenation even further than tetralin is said to not require expensive catalysts (mentioned in the other thread), e.g. GB147474 and 172688, or
much pressure.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
The preparation of nitronaphtalene is a simple nitration, the only thing is cooled nitration solution is needed, elseway the 2-nitronaphtalene will be
formed. Dissolve the naphtalene in ccH2SO4, add a mixture of 65% HNO3 and ccH2SO4 (1,5eqimolar), pour it on ice and woala. -lot recipes on the net.
The reduction should be performed with Fe+HCl -this is cheap  -there is a lot
recipe on the net, usually they make aniline from nitrobenzene with this method -there is a lot
recipe on the net, usually they make aniline from nitrobenzene with this method
1-aminonaphtalene could be hydrogenated to the corresponding tetralin with sodium and pentanol, this method is described by:
Green; Rowe
Journal of the Chemical Society, 1918 , vol. 113, p. 968
The removal of the amino group could be done by diazotateing it (0-5 celsius) with some sodium nitrite and then react the corresponding diazo compound
by hypophosporous acid or some mild redusing agents.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Naphthylamines are known to be carcinogenic, beware!!!
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
I'm still alive, and I have prepared a lot kind of them
Just't don't eat them and there will be no problem
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
And rightfully so! Avoiding to UTFSE and not reviewing previous discussions on the topic is a deadly sin.
http://www.sciencemadness.org/talk/viewthread.php?tid=18756
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I see. Well, actually, no I don't. I have access to none of those texts, but judging from the ingredients required (Palladium on carbon? Might
actually be doable if someone explains it to me) most of these are beyond my reach.
And yes, my fundamental goal is tetralin.
kristofvagyok: So from what I'm understanding, the route described is thus:
1) Dissolve napthalene in pure, concentrated sulfuric acid. Then add a 1:5 molar mix of concentrated sulfuric and nitric acids.
2) Pour it on ice (what does this do? Precipitate out nitronapthalene?)
3) Reduce with Fe + HCl (need more explaining on this part. Amounts to use, which to add first (or both?), what do I need to recover here (liquid or
precipitate, if any)?)
4) Hydrogenate with sodium and pentanol (see above)
5) React with sodium nitrite and then hypophosphorous acid (where do I get these?)
All in all, my original plan was seemingly to reduce molten napthalene with ethanol and sodium. But, I don't know if that works or if it produces a
pure product.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
"Ingredients", "recipes"? Cookmadness.org? I hate to be a symantic nazi but let' try using words like "reagents", and "procedures" this is
sciencemadness.org after-all.
Element collector you might want to read up on nitration reactions before attempting to do 'step' 1&2. You should also look understand doing an Fe
+ HCl reduction requires and over-head stirrer because of how magnetic stirrers work. To learn more about that step see nitro-benzene to aniline
reductions, in that case tin metal can be used instead.
Buying pentanol, NaNO2, or hypophosphorous acid to do this seems like a big waste of cash. Good luck getting a hold of hypophosphorous acid last I
heard it was very watched and pretty dangerous. Use the search engine and check amateur suppliers to find the nitrite. Keep in mind safety, this type
of chemistry isn't practiced without a certain level of hazard especially for the unexperienced.
[Edited on 13-9-2012 by smaerd]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | | I have access to none of those texts, but judging from the ingredients required (Palladium on carbon? Might actually be doable if someone explains it
to me) most of these are beyond my reach. |
Honestly, if you don't have the basic skills in employing references, or find trivial reagents being beyond your reach, most likely you don't yet have
experience with practical organic chemistry. For this reason, you truly should first practice on literature work and do a few basic preparative
syntheses before you attempt anything so hazardous. Not only are the Birch reductions with sodium in alcohols potentially dangerous, even reactions
like nitrations and reductions with iron easily go in a runaway mode if done at a scale larger than a about 50 mmol. If you intent to do experiments
on a 10 mmol scale, then fine, but don't go upscaling them before you become well experience. Even with enough experience, the thermodynamic aspects
of any reaction must be at least roughly researched before an upscaling is attempted.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
To be honest, I'm an inorganic chem person, so I kind of expected to be completely lost. However, OTC sources of tetralin are nonexistent as far as I
can tell, and I really think it's necessary for the one experiment I need it for.
As for employing references, I simply don't know how to access them. If I knew, I would.
...Millimoles? Oh dear. That's... not the scale of experimentation I'm used to.
I think I might have to scrap this experiment, and figure out how to buy the stuff. This turned out to be way more difficult than I expected, and the
reactions and chemistry are going completely over my head. Oh well.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
| Quote: |
textthe amides what got are quite explosive when they contact with water and it is really expensive.
|
one drop of sweat will do it in birch reductions, many people wind up on burn wards not understanding this fact, until later on.
Give me librium or give me meth!
Patrick Henry....
|
|
|
Dave Angel
Hazard to Others
  
Posts: 128
Registered: 22-3-2005
Location: UK
Member Is Offline
Mood: 0 K
|
|
Almost Tetralin...
This may be a long way round but it uses OTC/RA materials, of which I'm an advocate. The question is - would it work?:
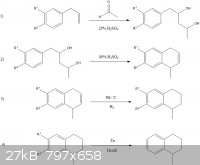
To summarise:
1) Take a phenylpropene [1] (in order of preference) chavicol, estragole, eugenol and perform a Prins reaction with acetaldehyde. In this case, the acetaldehyde would come from metaldehyde, either directly, or as a result of the
depolymerisation of the latter to the former in reaction conditions... hopefully (caveat #1).
2) In concentrated sulphuric acid, the product from the previous step undergoes elimination of water and (caveat #2) presuming some alkene is formed
on the terminal carbon then under the same conditions, a Darzens tetralin synthesis occurs. The position of the other alkene is abitrary as we'll be reducing this.
3) Nicodem states in this thread that a hydrogenation of dihydronapthalene to tetralin is achieved with hydrogen at atmospheric pressure with <1 mol% Pd-C, or
alternatively CTH conditions. Easy.
4) The final step is removal of the R groups. Distillation of phenol with zinc powder will yield benzene, so one can fairly safely assume similar
conditions will reduce any -OH groups on our aromatic ring. -OMe groups are another matter, as I'm not sure if the Zinc would kick them off at the
same time (caveat #3) or we need another step to take Ar-OMe to Ar-OH first.
So, a little long winded and full of caveats, but it was an enjoyable paper exercise regardless.
Pd-C might be the only challenge to obtain; perhaps materials for the CTH reactions [2] would be more readily available? Otherwise, everything is
fairly simple to acquire.
For extra credit: cash transactions for all RMs 
Seriously though, any thoughts on the chemistry?
[1] Originally I thought 'styrene!', but this came up one carbon too short. Thank the Gods for biochemistry and steam
distillation.
[2] Read of one here using PdCl2 / Formic acid / NaOH (aka. a trip the DIY shop and the jewellers), though this was for reduction of carboxylic acid
groups.
|
|
|