| Pages:
1
2
3 |
Chemtastic
Harmless

Posts: 31
Registered: 19-6-2004
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
I had an idea kinda like this, only the H2 and Cl2 were obtained seperately...
A deflated balloon would be stretched over the narrow mouth of a flask filled completely with vinegar (or other available acid) so that no air was in
either the balloon or flask. Inside the balloon would be a limiting amount of magnesium ribbon. It would be dumped, and a balloonful of H2 would
result...Mg + 2H+ --> Mg2+ + H2
Similarly, a deflated balloon would be stretched over another flask filled completely with vinegar, so that no air was present within. This balloon
would hold either sodium hypochlorite crystals or a strong solution of the salts. When inverted, Cl2 would result...2OCl- + 4H+ --> 2H2O + Cl2.
In this manner, balloonfuls of H2 and Cl2 could be generated.
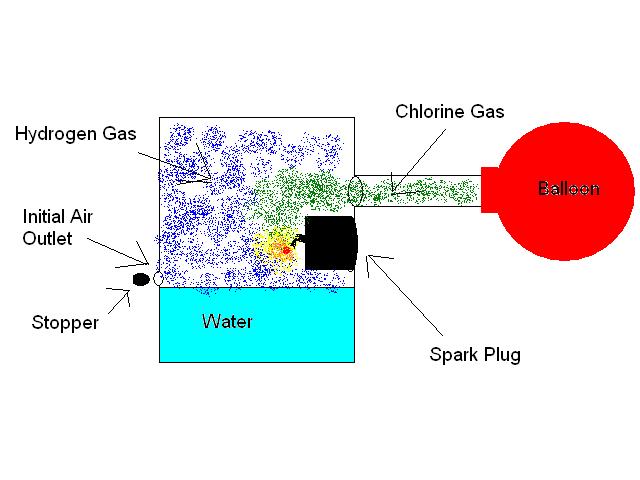
The apparatus would be something like this: A loosely sealed plastic container would have three additional openings on the side. The first would
accomodate a spark plug or some other sparking device and would be caulked in somehow as to keep the container airtight. The second opening would
have a narrow piece of plastic tubing leading from it, which would also be sealed in place, keeping the container airtight if the tube was blocked.
The third hole would also be on the side of the container, a small hole just above the waterline (the container is filled with that water that will
become acidic) used only in the pre-experiment setup.
Using the H2-filled balloons, air would be channelled down the pipe, sufficiently to fill the chamber and force all O2 and N2 out through the third
small hole just above the waterline. Once complete, this whole is immediately plugged. The container should now be airtight and have an exclusively
(or very closely so) hydrogen atmosphere within.
Next, Cl2-filled balloons are SLOWLY deflated down the tubing into the chamber. The sparker, set to spark at maybe 1-2 second
intervals, will not ignite the hydrogen gas, as no oxygen is present, until the chlorine gas enters. In order to minimize reaction intensity,
preventing explosion, the SLOW release of chlorine would be essential.
Anyway, this is my idea for an apparatus, and I am currently in the process of designing it. If anyone can spot any flaws, please let me know.
Otherwise, I'll try it and post anything I can about it...pictures, etc.
The attached picture is a JPEG of my proposed setup.
[Edited on 29-6-2004 by Chemtastic]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Just a minor flaw, but sodium hypochlorite does not have a solid form. You could easily bypass minor problem this by using calcium
hypochlorite(available as "unstabilized shock treatment" instead. Other
than that it seems viable. instead. Other
than that it seems viable.
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Actually, if Cl2 is collected in the balloon, why not just lead it through to dissolve in the water? A mixture of acids will result, with the
hypochlorous acids decomposed in the sunlight.
Additionally, making hydrogen gas by reaction with vinegar, is a teensy bit too slow as the acid is weak. You could try instead mixtures of warmed
NaOH and aluminium shreds. It would will give off H2 as usual, but with much more fervour.
Besides forcing out the oxygen out of the apparatus is hard unless, you have a certain chemical that removes oxygen from the air inside. Or instead of
sparking, why not use a UV lamp or bring it to the sunlight?
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Chemtastic,
Nothing wrong with the general idea, but I would like to make a few comments and sugestions:
1) Using magnesium to make H2 seems like an awful waste of materials. I wish I had some magnesium. I would go for Al and lye, like darkflame89
suggested.
2) I think Cl2 will attack the latex of your balloon fast. A balloon full of chlorine exploding in your face is the stuff nightmares are made of. Why
not use ziplock plastic bags? Take a look at this, I think you will love it:
http://mattson.creighton.edu/Part22-ZiplocBag/Part22-ZiplocBag.html
http://mattson.creighton.edu/AllGases.html
I used this technique to make some gases and it’s simple and reliable. Think of using large syringes and ziplock bags in your project.
3) I doubt you will be able to drive away all oxygen from the plastic container, so be prepared for an explosion at your first spark. This may or may
not be a great deal, just make sure the lid is loose. I agree with darkflame89 about removing oxygen, maybe an acid-wet steel wool hung on a wire.
Darkflame89,
Interesting what you said about chlorine in water. I wonder what would happen if I bubble hydrogen in bleach under UV light...
|
|
|
Chemtastic
Harmless

Posts: 31
Registered: 19-6-2004
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
Thanks, all. Just double checking one thing...UV light is enough for the activation energy of H2 and Cl2...is it enough for the activation energy of
H2 and O2?
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
If there is an acid-wet piece of steel wool in the container, won't it detract from the quality and amount of HCl produced?
To chemtastic, I think the balloons would work until they were eaten through, but it would be much easier to have everything connected directly and
not have to worry about balloons.
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
NO, UV rays arn't strong enough to initiate the reaction between H2 and O2. UV photon breaks up Cl2 to Cl free radical which attacks molecules
like H2 and O3 with full force..
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
Some positive observations
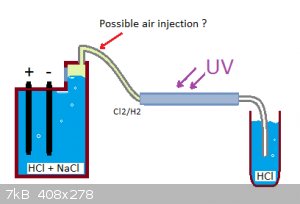
This experiment was tried as a scientific curiosity mostly, to see if H2 and Cl2 gas can combine "peacefuly" or not. Two carbon rods are electrolysing
a saturated salt solution at 2A and the two gases produced are collected together in a test tube through a bubbler. (You can see the setup in the
first attached image). What was observed:
1) The two gases can react slowly with low intensity light. Leaving the tube by the window in a cloudy day will result in half of the gases to be
consumed within a period of one hour. Leaving it in dark, would result in no gas consumption at all. In direct sunlight they would react within few
seconds but no explosion. As the reaction was going on, I could see the water under the test tube rising. For some reason, not all of the gas in the
tube could be reacted; probably because there was oxygen there too or because the Cl2/H2 ratio was not optimal (some Cl2 dissolved in the brine or the
bubbler water maybe). This deviation from optimal ratio maybe the reason I couldn't observe an explosion out of it. This may be a key observation for
designing a safe reactor in future.
2) As the electrolysis continues, NaOH is procuced. Within some minutes the solution is alkalic enough in order to be capable of consuming all of the
Cl2 produced and making hypochlorite. Thus we get only H2 reaching the output. The effect takes place as soon as the first minute has passed. One can
prove this by acidifying the electrolyte with HCl; Cl2 gas will be suddenly freed from the solution.
The title of the thread characterises the electrolysis way "ineffective" and "dangerous". There is a possibility by using carefully controlled UV
light instead of sparks to make the reaction less dangerous. Also, if a membrane cell is used and the NaOH does not come in contact with the Cl2, this
way could become much more effective.
Probably this device would never become practical enough for producing HCl, but here you have a proposed unit: (check second attached image)
If a separating membrane is not used, the solution must be kept acidic at all times by adding hydrochloric acid. In that way, it can only be used to
purify impure hydrochloric acid and not to produce it from NaCl.
|
|
|
MeshPL
Hazard to Others
  
Posts: 329
Registered: 20-4-2015
Location: Universe
Member Is Offline
Mood: No Mood
|
|
I had this idea for "safe" HCl generator for a while, it would probably need some cooling, but here is the rough idea. Not sure if it would work.
Obviously, the goal for this one is production of conc. aquous HCl.
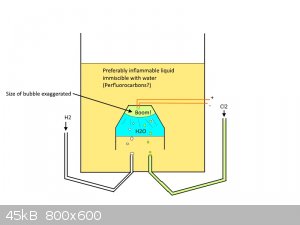
[Edited on 19-3-2016 by MeshPL]
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
It works
Thanks for the input MeshPL.
In my opinion, one should avoid the usage of sparks for combining H2 and Cl2 since a simple UV lamp can do the same much more safer.
If you decide to use the sparks, you will have the benefit that H2/O2 gases will react too. I think it is unavoidable not to get O2 impurities through
brine electrolysis. Using UV light in a closed system like the one you described is not practical (the inner chamber will fill with O2/H2), thus you
must use the sparks in that setup.
Usage of UV light requires an open system but it has other advantages. There is no need to separate H2 from Cl2 prior to the reaction (which means a
very simple and small electrolyser) and there is almost no explosion danger.
For the time being I don't fully understand how those two gases could combine explosively. My attempts to trigger the reaction with UV light are in
vain. The reaction proceeds smoothly even near a black light tube. I think a much more intense UV source must be used in order to trigger it, but I
don't care about that right now.
Bottom line is that the UV light method works. In one test, it was able to lower the pH of 50ml of deionised water around pH 2 only after 20 minutes
of operation. I know it is aqueous HCl in there since the water also acquires a sour taste. There is barely any Cl2 smell in the output gases wich
means that most of Cl2 reacts. Very promising...
I hope the next days I will come up with some serious measurements of efficiency and output capacity if you are interested. Until then, wish you guys
a happy experimenting on whatever you do.
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
Some news:
Finally, a violent reaction between H2 and Cl2 was observed when exposed to UV. In order for it to happen it requires purity; mix with the H2/C2
stream some air or other indifferent gas and you will not get it. However, the reaction to HCl proceeds smoothly even if the mixture is diluted with
air and that's a fine thing to see! But keep the tubing thin and use a protective box around the device, just in case.
Still not any rigorous measurements on efficiency. Best thing I can say is that with 1.1A for 20mins I got 50ml of HCl solution that was capable of
producing fizzing when mixed with soda. That is enough proof for me.
Now to the problems:
1) I noticed that the HCl solution has a smell like bleach. Obviously some Cl2 gets dissolved in there even if the pH is low 
Well, this is bad news for me since I'm interested in the purity. Does anyone know if it is possible to remove the Cl2 from the HCl? I'm afraid it is
difficult to do..
2) Still a solution on how to separate NaOH from mixing with Cl2 in the anode has to be conceived. Else, the device will not be able to work on salt
only, it will require acidification of the electrolyte too.
So, for the time being it is useless; cannot be used to purify HCl acid neither to produce it from salt. Any practical ideas on how to solve at least
one of those problems might kindle my interest again.
[Edited on 22-3-2016 by experimenter_]
|
|
|
pesco
Harmless

Posts: 20
Registered: 19-11-2015
Member Is Offline
Mood: No Mood
|
|
Just few ad hoc suggestions:
1)
a) use excess of H2
b) make sure your HCl collection vessel is under UV and bubble H2 though it on the way to H2+Cl2 reaction chamber
c) both above combined 
2) divided cell
Regards
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by pesco  | Just few ad hoc suggestions:
1)
a) use excess of H2
b) make sure your HCl collection vessel is under UV and bubble H2 though it on the way to H2+Cl2 reaction chamber
c) both above combined 
|
Thanks for the suggestions pesco. Both of them seem logical steps to take. I think (b) is the easier to do but also less possible to work.
My ideas on this was to use vitamin C or sodium ascorbate to react the chlorine towards HCl (according to this link: http://www.fs.fed.us/t-d/pubs/html/05231301/05231301.html). The sodium ascorbate will be positioned after the UV stage but before the water for
the bubbling of HCl. In that way, chlorine will never reach the water to dissolve to; it will be reacted while it is still in the tube. Hopefully, HCl
gas does not react with sodium ascorbate or else that way will not work. Anyway, I have three methods to check now, thanks to your input.
Good news for this one. I have already found that two half-cells connected with "salt bridge" made from ordinary filter paper works just fine. They
produce separately H2 and Cl2 gases which are mixed in a later stage before the UV chamber. The rate of gas evolution is almost not affected by the
"salt bridge"; it can carry 1A easily if elevated voltage is used to overcome the resistance. Added bonus is the production of NaOH also. I don't know
yet what the maximum concentration of NaOH can be with a lengthy electrolysis.
I didn't want to use membranes that need to be bought from special shops. After all it is just some HCl that is produced. It must be made by the
simpler possible way.
So, issue No2 is solved and issue No1 has a big probability to be solved easily too. When I get in the mood, I might dedicate a new thread to the
device and take some pics; it is not very pretty but it is a piece of art in a way that only a chemist can appreciate 
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
here to necro a thread back to life with my experiment on this.
IT FKING WORKS GUYS
its not explosive if the chlorine is wet! however dont pass too much at a time.
UV reactor setup
https://www.youtube.com/watch?v=Hssf-_z_O8s
HCl test from setup
https://www.youtube.com/watch?v=2DR-nPLFvZU
im wearing anti UV welding goggles so dont worry about my eyes.
Below is my chloro alkali PVA type sodium ion exchange membrane which produces an equimolar Cl2 and H2 mixture which if passed thru water safely wetts
the chlorine so it dont explode!
I only have to buy salt and I can produce fairly concentrated HCl!!

[Edited on 11-9-2020 by mysteriusbhoice]
[Edited on 11-9-2020 by mysteriusbhoice]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
cost of production using my method of membrane cell + UVC is 82 cents per liter of 37% HCl so I would say its dangerous but not expensive at all nor
inneficient.
|
|
|
khlor
Hazard to Self
 
Posts: 82
Registered: 4-1-2014
Location: Who knows, really...
Member Is Offline
Mood: No Mood
|
|
Alright... I might just have dived into crazy territory here, but I've read on a big yellow chemistry book here that the H2+Cl2 reaction is
facilitated at a temperature of over 250C, plus, on wikipedia and other places list Cl-Cl bond to require over 2.51eV to be broken, so I did some
digging and in theory, you can use blue and violet leds to do it, since anything bellow the wavelengh of 450nm should provide enough energy in(eV) for
that(I have some 3W UVA leds, not tested yet though) however, what I did was use an undervolted halogen lamp to reach the said 250C and turns out it
works pretty fine, and for my surprise, no explosions(I was expecting such and PREPARED for such) nd I saw the pure HCl vapor comming out of the
reactior as it formed a mist by absorbing the air humidity... kinda neat, and scary. then again, I turned the lamp on first, before turning on the
gas, to make sure that all mixtured that got into the chamber reacted immediately.
"NOOOOOO!!! The mixture is all WROOOOOOONG!"
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Why not just use chinese quartz glass UVC bulb to catalyze the reaction? They cost few € a piece for 10-30W bulbs.
I actually bought one just for this specific chloro-alkali cell, but as I never got it working, I later dismantled the operation.
Would be much more controllable and economical than using spark plug. The reaction setup could be contructed such that the reaction chamber is
buffered with something like mentioned carbon, and lined with material that is transparent to UVC when necessary, and otherwise opaque, to prevent it
reacting the gases prior. The gases should also be led through different lines to the chamber. As it is energetic, the chamber should have water
jacket. Perhaps a quartz insert tube into an ordinary multi-neck boro flask immersed in water bath with circulator pump to move water around?
Scattered UVC would be enough to radicalize the reactants within.
IMO balloons and other static housings for gases are conceptual at most. Having a mass of 2g/L or likewise, one would either generate only grams of
results minus losses. Imo a chloralkali cell is so laborous to construct that it would mandate the production of at least kg scale of reactants to be
anyway excuseable. Good old bisulfate+salt is several orders of magnitude lower in lead-in costs and labor.
[Edited on 1-2-2021 by Fyndium]
|
|
|
| Pages:
1
2
3 |