| Pages:
1
2
3
4
5 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
If it was colloidal it would probably run through the filter, like a solution. Fe(OH)3 has a tendency to do that and I've seen it happen: allof a
sudden your precipitate ends up at the wrong side of the firlter, very annoying. But that didn't happen here... It's called peptisation. 'Sn(OH)4'
does it too.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
@ blogfast:
I think he would be better to use my procedure, more straight forward and more robust not to mention simpler.
He seam to have some difficulties with the peroxide method.
I never asked for this.
|
|
|
Salmo
Harmless

Posts: 42
Registered: 20-9-2012
Member Is Offline
Mood: No Mood
|
|
Man i really dont know what it was, I just can tell you that it was a really thick sludge that wasn't forming any kind of precipitate, it was more
like a fucking gel impossible to vacuum filter. Anyway as I'm bored to use nasty HCl at home  , tomorrow I will electrolyze a big spoon in a NaCl solution, I did it before with good results, then I will precipitate everything with
NaOH, add peroxide and than filter the mass to obtain the chromate solution, i will post photos! , tomorrow I will electrolyze a big spoon in a NaCl solution, I did it before with good results, then I will precipitate everything with
NaOH, add peroxide and than filter the mass to obtain the chromate solution, i will post photos!
Plante your procedure is for sure better and faster I have to obtain some sodium nitrate first, can yuo tell me your exact procedure?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by plante1999  | @ blogfast:
I think he would be better to use my procedure, more straight forward and more robust not to mention simpler.
He seam to have some difficulties with the peroxide method.
|
To make such a statement a robust comparison between the methods would need to be made first. I started of with some kind of fusion (KOH + KClO3),
only to find it wasn't necessary: everything can be done at RT and in solution/slurry.
An example of separating the Cr from an FeCr heating element, here:
http://www.sciencemadness.org/talk/viewthread.php?tid=21298#...
Still, why not post the details of your [plante] approach here?
[Edited on 1-10-2012 by blogfast25]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
You have it right on the point that I should upload it, I would prefer to do a prepublication about it tough. I tried both and found the peroxide way
a lot harder to do because of colloidals, concentrations of solution not to mention a lot more costly than hydroxide melt.
I never asked for this.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Well, let us know when the prepublication is up.
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by plante1999  | | I also used electrolysis of S.S in a carbonate electrolite but I found it energy intensive and take time to make some chromate but Hasslefree.
|
sounds interesting..do you have any more details on this? do you add any oxidizer to the electrolyte?
i just did the potassium version of this. after filtering out the largely unreacted Cr2O3 i got a yellow solution that i'll
quantitate asap.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Only electrolysing S.S at 20V in Na2CO3 electrolyte. Sodium chromate go in to solution but iron and nickel don't. It take a large amount of time to
get a product but it work really well. If molten for enough time all the Cr2O3 will react. Use stochiometry to know hydroxide/nitrate ratio.
I never asked for this.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
If you have an oxidizer such as KNO3 or KClO3 on hand, you can perform a fusion:
http://webpages.charter.net/dawill/tmoranwms/Chem_Chromate.h...
The KCl and NaOH aren't necessary; with KClO3 at least, the reaction proceeds under "acidic" conditions, yielding KCl and K2Cr2O7 directly (but beware
the fumes, exotherm and decomposition).
This may not work with ashed* stainless, because nickel or iron are probable catalysts for decomposition. I once tried the reaction on mixed oxides
where chrome was known to be present; all I got was frothing (decomposition, O2) and a lot of black stuff.
It's noteworthy that molten KClO3 is reasonably stable in a steel or stainless crucible.
*By 'ashed', I mean oxidized, dried, powdered material. It could be burned in air (well, if you can about melt the stuff; it doesn't oxidize very
fast otherwise), dissolved in acid and precipitated wholesale (mixed hydroxides), calcined, etc.
On a related topic, I seem to recall PbO2 can also be made by a similar method, but I'm not set up to test it.
Tim
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hi Tim,
It's quite similar to treating Chromite (FeCr2O4) with KOH/KNO3, then leaching. The iron stays, the chromate is lixivated off. Nice page!
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
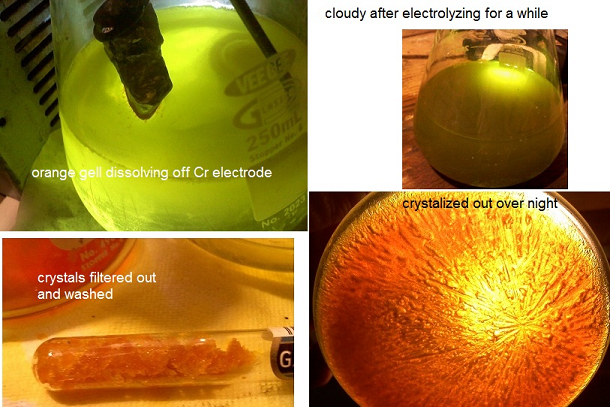
so I have been messing around with the idea of purifying the chromite ore I found, but along the way just wanted to see if I could make Na2Cr2O7 from
pure Cr metal. and I think I have come up with a pretty darn easy way.
I made a decently strong NaOH solution and immersed a ~99.1% chunk of ebay Cr metal(+) electrode and a Ti(-). the solution turns bright yellow
after a short while, because of deep orange gel pouring off the Cr electrode. this worked so far... the colors were right. I figured ok let it run a
while build up a concentration. it developed a darker(greyish) cast and became more opaque. My only guess was that there was no longer any sodium to
keep up with the dissolving Cr. there were some small fragments of Cr metal so I figured maybe I can add H2O2 and or lye to see if I could oxidize it
after providing more Na ions. both caused bubbles and heat. after filtering w/ coffee filter I found a grey gel with the tiny bits of Cr glimmering
in it, and a BRIGHT yellow clear solution. this was good for the chromate I thought, and after adding a small amount of H2SO4 drop-wise to the
solution it became decidedly orange! again I figured I was on the right path. the H2SO4 didn't noticeably fight with NaOH as no bubbles were
evolved. figured this meant the sodium was used up.
I put this flask in a water bath just below boiling, and waited patiently. went from 125ml --> ~50 ml before I noticed anything. orange
crystals at the edge of the liquid. filtered it around 30ml and got a decent amount of orange crystals. though they don't seem to be too regular.
need to recrystalize after washing w/ ice water. my 12th merck said that sodium dichromate aq. can be set to ~100 for a prolonged time to make
anhydrous. I deff had crystals and didn't see any breakdown, and the onely elements I introduced were Cr, NaOH, H2O, with a bit of H2SO4, and H2O2.
so given the behavior and colors I figure I have Na2Cr2O7  though tired now and
ready for bed 6:30am... though tired now and
ready for bed 6:30am...
but seems pretty simple to use NaOH sol. and slap a Cr electrode in there. then throw a small amount of juice at it. my cell(250ml Erlenmeyer) needed
to be put in a buffering water bath. not cooling but to tame against thermal shock. my power supply was an OLD hacked ATX computer spare.
maybe tomorrow I can figure out how to get some pic's up. gotta buy some more gloves also. if some one thinks I may have performed some other
reaction then please enlighten me. but I feel fairly confident. if it did work I don't see why this couldn't be adapted to the use of Stainless
Steel. though I would stay the heck away from HCl with this stuff. chromyl chloride isn't for me.... I know that in the test run of this I used a
stainless nail to make contact to the Cr metal. the Fe contamination just turned to a brown gelly at the bottom, and man does Ni drop out of solution
with NaOH in there. ( just got done making nickel chloride, sulfate, acetate and hydroxide last week). any thoughts?
[Edited on 13-10-2012 by violet sin]
|
|
|
Salmo
Harmless

Posts: 42
Registered: 20-9-2012
Member Is Offline
Mood: No Mood
|
|
Ok finally I did it!
I told you that I would have posted my procedure and the photos so here I am.
I started from 18/10 stainless steel from a fork, I electrolyzed it in a 25% NaCl solution with my lab power supply adding some 33% HCl to destroy
the hydroxides formed. than I stopped it after 2 hours and I filtered the solution to obtain a clear green solution.
Than I added a saturated Na2CO3 solution to precipitate iron/nickel and chromium as hydroxides, I didn't use a NaOH solution because strong sodium
hydroxide solutions react with chromium hydroxide to form soluble [Cr(OH)6]3-.
Anyway after the precipitation I filtered the solution and I obtained a dark green/brown sludge that I washed with water. Than heating the sludge, I
added a really strong NaOH solution to it,and than 35% H2O2, 10ml a time, to oxidize Na3[Cr(OH)6] to sodium chromate, so the sludge after lot of
fizzing, turned brown and I filtered it obtaining a yellow sodium chromate solution.
So because I wanted to remove the excess of NaOH, NaCl and everything else I added HCl to the sodium chromate solution until acid, I reduced it adding
hydrogen peroxide again and I precipitated Cr(OH)3 adding sodium carbonate.
I washed my chromium hydroxide with hot water many times and I obtained a blue mass that I redissolved with as little as I could of a strong NaOH
solution than I added the right amount of H2O2 and I heated the solution, after filtering I got a really strong yellow sodium chromate solution.
Now I checked the pH of the solution and it is high so I have some sodium hydroxide in it, do you know how can I remove it for the last time?? I
thought to sulfuric acid until pH 7 maybe sodium sulfate is easy to remove.. I dont know
Damn I want an induction heater and damn believe me filtering hydroxides or oxides is time consuming and a pain in the ass.
        
[Edited on 13-10-2012 by Salmo]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Well done, Salmo.
Two comments:
Quote: Originally posted by Salmo  |
Than I added a saturated Na2CO3 solution to precipitate iron/nickel and chromium as hydroxides, I didn't use a NaOH solution because strong sodium
hydroxide solutions react with chromium hydroxide to form soluble [Cr(OH)6]3-.
[snip]
Now I checked the pH of the solution and it is high so I have some sodium hydroxide in it, do you know how can I remove it for the last time?? I
thought to sulfuric acid until pH 7 maybe sodium sulfate is easy to remove.. I dont know
|
Your reasoning is correct but chromite [Cr(OH)4(-)] does not appear to form when large amounts of iron are also present (as is the case here). In
those circumstances, the Cr3+ appears to co-precipitate fully with the iron oxide(s). But as you’ve seen later on, Cr(OH)3 on its own does dissolve
in strong NaOH to form sodium chromite.
Re. removing the remaining peroxide. DO NOT acidify at this point because in acidic conditions the excess peroxide will reduce the chromate back to Cr
(III)! The best way of destroying left over hydrogen peroxide is to simmer your solution for some time. Then take attest tube sample and acidify it.
It should revert to the yellow/amber of dichromate. If any blue/green appears, you’ve still got peroxide left.
Re. removing the excess NaOH. For dichromates usually the K salt is preferred, I believe because K2Cr2O7 is only sparingly soluble in cold water and
thus easier to isolate. The excess KOH would be neutralised with H2SO4 and on cooling crystals of K2Cr2O7 would appear, provided you started from
about the right amounts of KOH and Cr. I’m not sure how this will pan out with Na2Cr2O7.
Nice photos!
[Edited on 13-10-2012 by blogfast25]
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
so was there anything wrong with the route I took? I don't have Ph test strips and reagents are quite limited.
the crystals aren't defined well in the cigar tube b/c I didn't have time to do anything but give 'em a quick rinse in the filter. were there any
concerns of contamination? other than Na2SO4? blogfast25 you said something about soluble chrome hydroxides but not to worry in an alkaline setting?
just been having fun with the electrochem and a few of the metals I collect.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
VS:
Is that sodium dichromate or potassium dichromate in the cigar tube? To purify further, recrystallise.
Nice work!
Re. Cr(OH)3 and chromites, see further upthread...
|
|
|
Salmo
Harmless

Posts: 42
Registered: 20-9-2012
Member Is Offline
Mood: No Mood
|
|
Thanks blogfast today I will acidify drop by drop with sulfuric acid to ph 7 and than I will try to separate the Na2SO4 using its different
solubility.
Na2SO4 is 4.9g/100ml ( at 0°C) and sodium chromate is 163g/100ml ( at 0°C).
Violet sin, I think you have my same problem, the fucking sodium! anyway good job now recrystallise.
Check this site: http://www.chemguide.co.uk/inorganic/transition/chromium.html
[Edited on 14-10-2012 by Salmo]
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
blogfast: thanks that is the Na salt. obviously not pure as I am reading in "atboic:Cr" from the reference section right now. thought the stuff in
the cigar tube is from the first stuff to fall out from water bath reduction, and more likely pure(er)...
facing Cr(OH)3 and the chromites it makes with NaOH... gotcha
from what I gather you are saying, starting off with a strong NaOH sol for electrolyzing means I have the Na3[Cr(OH)6] and Na[Cr(OH)4] from the moment
Cr oxidizes into solution? -but- it can be oxidized from the -ite to the chromate with H2O2 in it's alkaline situation. then further boiling I would
get rid of extra hydrogen peroxide, before H2SO4 acidification to the dichromate( which I wanted for conversion ). but I only really have a bit of Na
sulfate salt contaminant from neutralization of excess NaOH *IF* I managed to get all of the odd hydroxide complex completely oxidized to chromate
first *AND* I boiled it long enough to kill the H2O2 before acid so it doesn't revert to the chromite correct?
the grey gel I had with the sparkly bits in it may be the hydroxide 'aging out', and as a result of Cr3+ from adding the H2O2 in acidic conditions?
explains why it got cloudy a few times( playing with H2O2)
or is there still a chromite that is not solved by oxidation to chromate with H2O2? I only have 3% so it is a LOT of fun to add and boil down. if
there was any left over then it is surely gone by the time I through with it's water... those older books are kinda hard to get the hang of,,
having to rearrange stuff in your head for hydrated forms or odd oxides that are a mix of two other oxides. I say if you haven't been doing that for
a while it's a real treat to try and keep it all straight
thanks also salmo nice info site. like it. I think the reason for doing it in Na form is the merk says it will be fine to dehydrate --> anhydrous
by water bath or @ ~100'C for extended time w/o decomp probs. then it would be easy to add water necessary and do the KCl dance quickly. that's my
end goal with this exploration, no reason just looks fun. I just wish I could accidify it with something that didn't have a fun salt leftover like
Na2SO4
[Edited on 14-10-2012 by violet sin]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Chromates are very strong in color and stain everything, so you could have sodium sulfate with surprisingly little (di)chromate. Not sure if they
form solid solutions; I suppose chromate (but not di-) could partially substitute sulfate.
One easy way to tell, take some crystals and attempt to dry or melt them. Sodium sulfate hydrate decomposes under low humidity conditions, or melts
in its water of crystallization, IIRC around 80C.
Precipitating with potassium has the downside that K2SO4 is also fairly low solubility. Ditto ammonium.
Using HCl instead of H2SO4 would be better; both KCl and NaCl have good solubility at all temperatures.
Tim
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
well it would be hard to have too much Na2SO4 in there b/c I only had to use a few ml of H2SO4 to acidify to orange, whereas my chromium metal chunk
went from a nice angular shattered look to a well worn beach pebble. I think in the entire 250ml flask I used ~5ml for shift(<10ml, was only a 1ml
eye dropper). that leads me to believe that I used up most of the NaOH forming the sodium chromate and chromite that got oxidized so ya chromate( as
I used 4-5 Tbs. NaOH). I stopped energizing once I noticed a greyish opacity(Cr(OH)3), then filtered. that was more than likely the solution ppt out
Cr(OH)3 because of lack of free OH- to solovate. then oxidized and acidified. so I was deff on the right track but had the hydroxide/chromite like
blogfast n salmo said. not saying you are wrong just I don't think I had a lot of sulfate...
looking back after the last few very helpful posts, I dissolved everything again this morning and added H2O2 back to clear yellow. only a small pile
of clean Cr crumbs were left in the eddy after much bubbling and quite a few additions. seemed I had a rather sizable amount of Cr(OH)3/chromite. I
left it to simmer @~90'C for like an hour to boil down and kill H2O2.
I did notice when dissolving crystals again that something was wrong, took more water & time than it should have, so quite glad I cleaned it up.
I like the dry melt idea, shoulda looked up more physical traits to compare before declaring DONE. good point. no HCl for me with Cr, afraid of the
chromyl chloride. I have less than ideal ventilation... no thanks. working on a dimmer-switched vent fan/hood and ducting today as well.
construction background comes in handy yet again 
trying to get some pictures up for re-polished product soon. also started a small batch from stainless nails. doing everything salmo did just on a
50ml scale just for shitts and giggs. salt works surprisingly well for devouring SS
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Yup, if there's a lot more chrome than sulfate then it's probably not going to show up.
Chromyl chloride isn't a concern, as far as I know: it hydrolyzes rapidly in water. A bigger concern would be dichromate oxidizing chloride to
chlorine and reducing your yield, but I think this is unfavorable too, and in any case not a problem if an excess of acid is not added.
Tim
|
|
|
Salmo
Harmless

Posts: 42
Registered: 20-9-2012
Member Is Offline
Mood: No Mood
|
|
Today I will try to purificate my sodium dichromate solution removing all the sodium sulfate inside, i will boil down the solution to 10ml than I will
put it in the fridge to 0°C hoping to see some sodium sulfate precipitation but anyway it's hard that i will obtain something pure..i think that
solid fusion is the best idea because it's so difficult to add the right amount of NaOH during the oxidation and adding too much means a not pure
product full of NaCl or Na2SO4..
[Edited on 15-10-2012 by Salmo]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes, separating the chromate/dichromate from neutralisation by-products may prove to be the hardest part here.
Obviously, however you want to make these Cr (VI) salts, the trick is to keep by-products to a minimum by working as close to stoichiometry as
possible. Small amounts of soluble by-products should then always remain in solution. So as with any decent synthesis, planning ahead is crucial.
Tim’s right about the solubilities, KCl is somewhat more soluble than K2SO4. HCl also has the advantage that it’s volatile so that any excess will
evaporate on drying your end-product.
The solubilities for the Na and K dichromates at 0 C (Wiki solubility table) are resp. 163 [that sounds really high!] and 4.7 g/100 ml, so for
dichromates the alkali of choice should be KOH,H2O2 as oxidiser and HCl as neutraliser. Unless you want to use fusion.
[Edited on 15-10-2012 by blogfast25]
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by plante1999  | | Only electrolysing S.S at 20V in Na2CO3 electrolyte. Sodium chromate go in to solution but iron and nickel don't. It take a large amount of time to
get a product but it work really well. |
i've been running this the whole day, using a chrome plated stainless steel fork for anode and a graphite cathode. the chrome plating resisted
corrosion so well that i had to temporarily hike the voltage to almost 40V before the electrolyte caught color (a previous addition of
H2O2 didn't help). i'm finding it hard to keep the voltage that high as my PSU maxes out at 6A. i had to increase resistance by
reducing the anode surface dipping in the electrolyte, but this feels wrong from an efficiency standpoint. does it necessarily have to be that high to
oxidize the Cr to +6?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Hmm, K2CrO4 has higher solubility than K2Cr2O7. Might be useful. pH swing crystallization or something? Take your impure mixture, add an estimated
stoich amount of KOH, wait to dissolve; evaporate to saturation, then add HCl. KCl and NaCl stay put (unless they are also at saturation, in which
case the added chloride may drop a small amount; a second pass should be clean).
And, of course, collect all wastes and crystallize out all the orange and not-orange stuff and repeat...
Tim
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Quote: Originally posted by tetrahedron  | Quote: Originally posted by plante1999  | | Only electrolysing S.S at 20V in Na2CO3 electrolyte. Sodium chromate go in to solution but iron and nickel don't. It take a large amount of time to
get a product but it work really well. |
i've been running this the whole day, using a chrome plated stainless steel fork for anode and a graphite cathode. the chrome plating resisted
corrosion so well that i had to temporarily hike the voltage to almost 40V before the electrolyte caught color (a previous addition of
H2O2 didn't help). i'm finding it hard to keep the voltage that high as my PSU maxes out at 6A. i had to increase resistance by
reducing the anode surface dipping in the electrolyte, but this feels wrong from an efficiency standpoint. does it necessarily have to be that high to
oxidize the Cr to +6? |
Like I already said, It take time is inefficient , but It work. If your S.S is hard to dissolve use Sodium bicarbonate electrolyte, It should help for
the dissolution of the Chromium. The idea is that the Ph is lowered increasing corrosion of iron.
I never asked for this.
|
|
|
| Pages:
1
2
3
4
5 |