Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Is this reaction possible?
I've been trying to read up on ways to introduce a carboxylic functional group to a weakly deactivated
aromatic ring, in pursuit of a method of synthesizing luminol from PET bottles that have been converted to benzene-1,4-dicarboxamide.
Using http://en.wikipedia.org/wiki/Nucleophilic_aromatic_substitut... and http://en.wikipedia.org/wiki/Deactivating_groups as references I've thought up the mechanism below.
Does it seem plausible? And if not, is there another method which I can use to introduce such functional groups and not risk reactions that would take
place with the other functional groups on the aromatic ring, without the need for use of protecting groups?
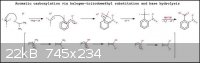
Explanation of mechanism.......[From left to right]:-
1). Iodoform is deprotonated in an alcohol solution of equimolar sterically hindered, metal alkoxide which reacts to form the triiodomethyl anion and
an alcohol molecule (meanwhile the metal cation acts as the counterion to the anion formed).....{If the molar ratio of iodoform to alkoxide is not
equimolar then the iodoform should be present in the larger molar proportion}.
2). The triiodomethyl anion then attacks the carbon directly bonded to the halogen atom and forms a bond to the carbon. This causes electron density
to be pushed from the carbon with the halogen substituent to the carbon in the ortho position with the electron withdrawing group. Due to the change
in electron density in the electron withdrawing group it then rearranges to compensate for the change. In the process the aromatic ring loses its
aromaticity and thus its stability.
3). To regain aromatic stability, the excess electron density in the EWG is withdrawn and is pushed into the better leaving group attached to the
primary carbon atom, in this case the halogen atom iodine, forming the iodide ion. The iodide ion pairs with the lithium countercation and leaves the
reaction mixture.
4). The resulting triiodomethyl-nitroarene molecule is then hydrolysed in base to the unstable orthoformic acid-nitorarene derivative and iodide
byproduct. The orthoformic acid group then decomposes to produce the carboxylic acid-nitroarene and water. (The orthoformic acid-nitorarene derivative
formation and decomposition was omitted).
Please reply telling me what you think of this reaction.
Thank You......
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
I think that won't work.
First reason: iodine, especially here the iodoform doesn't react like this. It is not a really strong acid, and if you react it with isoropoxides it
will produce orthoformates, so you will end up here with triisoproyl orthoformate and some LiI in solution.
Second. The iodine atom is large, really large, so if you are willing to substituate anything on an aromatic ring than it should have enough space.
The triiodomethyl group won't fit there.
And there is another thing: there is a Reimer-Tiemann reaction what goes with chloroform and NaOH/KOH, but it usually works well on activated aromatic
substances e.g. phenol.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Revision of mechanism
Oh dang! I was hoping that it might have had at least a slight chance of working.
About the use of isopropoxides. I wasn't planning to use secondary alcohol alkoxides, just was doing something of a generalization. I was planning to
synthesize a bulky alcohol alkoxide molecule like lithium dimethyl-phenyl-pentoxide which would hopefully, due to steric hindrance, be unable to form
the orthoformate esters.
Please remember that I said I was dealing with deactivated aromatic rings, therefore the Reimer-Tiemann reaction most likely would not work in this
case since a step in the mechanism involves pushing electron density from the activated aromatic ring to the dichlorocarbene. Since the aromatic rings
will be deactivated then this important step would not be able to proceed.
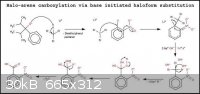
Okay, so above is my revised reaction mechanism utilizing bromoform instead of iodoform. I was hoping that since bromine is more electronegative than
iodine then that would mean that there would be a greater possibility for the formation of the tribromomethyl anion, due to the bromine's better
electron withdrawing nature.
About the iodine. I really didn't think about the iodine's size and the size of the replacement substituent. I didn't realize that the sizes would
play such important roles in the determination of the mechanism's feasibility.
Considering sizes, I think iodine would still be a good atom for replacement because it is a very good leaving group. However, since you highlighted
that the SN2 step of the mechanism in which both substituents are attached to the carbon could pose a problem, I'm wondering what would
happen if I also utilized a stoichiometric amount of cuprous iodide in the reaction mixture.
I'm thinking that the CuI would form a complex or an adduct with the haloarene molecule which would make it easier for the iodine atom to be replaced
be the tribromomethyl anion and in the process the CuI could react to form the complex anion [CuI2]- with the counterion being
the alkali cation Li+. The conversion of the CuI to Li+[CuI2]- would be what helps in making the process
go forward in favour of the formation of the trihalomethylarene derivative (maybe, just a thought).
Possible experimental conditions and procedure:
An anhydrous polar aprotic solvent is selected which can dissolve all the chemicals. The compound to be carboxylated is dissolved in the solvent and
the bromoform and cuprous iodide are added (with the bromoform in slight molar excess). The solution is thoroughly mixed and is gently warmed. A given
amount of alkali alkoxide is dissolved in fresh solvent and is thoroughly mixed. With stirring, the alkoxide is added dropwise to the primary
mixture. After complete addition of the alkoxide, the solution is refluxed for about an hour. An aqueous solution of base (in excess) is then added
slowly to the boiled reaction mixture and after complete addition the mixture is then heated and stirred. The aqueous layer containing the carboxylate
salt is separated and acidified. The resultant carboxylic acid precipitate is then filtered and purified.
To anyone who would like to help, your input would be greatly appreciated.
Thank You......
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Exploratory experiment time
Well, I guess it is experiment time.
I've devised an experiment by which I can test the feasibility of this reaction, and I will test it on different deactivated halgenoarene substrates.
If everything goes as planned the halogenoarene compound will be converted into a carboxylic acid.
Below is the proposed experiment, utilizing the substrates 1,4-diiodo-2-nitrobenzene and 2,4-diiodobenzoic acid, produced from poly(ethylene
terephthalate) bottles.
If the reaction works then the first carboxylic acid to be synthesized will be 4-iodo-2-nitrobenzoic acid. The second carboxylic acid to form will be
4-iodobenzene-1,2-dicarboxylic acid. The 4-iodobenzene-1,2-dicarboxylic acid can then be dehydroxylated to phthalic acid, which can be used to
produce luminol.
I will conduct these reactions and update this thread as such.
Thank You......
"Discoveries are not made by idly sitting around and hoping something interesting might happen; they are made
by getting out there and doing something to push the results and odds in your favour." "Chemistry always works... just not always in the way you
want."
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
What in the hell is going on in that reaction scheme. There are several threads on this forum concerning the isolation of phthalic acid in vastly more
straightforward (and for that matter, possible) ways.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I wonder if an alcohol substituent could be added to the first position and then oxidized...although the deactivated ring could cause problems.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I can relate to where he's at. It's easy to get caught up in the end-goal from a certain way rather then the practicality and feasability. Sure PET
bottles are cheap but do you really want to be doing all that? Especially considering a lot of those reactions even IF it worked wouldn't be very
yield worthy.
There are a lot of flaws in this reaction mechanism. Hydroxylation of the iodobenzene functional group won't be going off without a hitch with NaOH.
The second to last step, the removal of the phenol functionality with an acid will not work. Not sure what the Li-O- ligand is? Not sure if it really
even matters.
I could try and sort through more of it but from a practical stand-point you might as well start from ortho-xylene and just oxidize to pthatlic acid.
Then make the anhydride. Much cheaper and higher yielding with a whole lot less leg-work. Plus it's documented  . .
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Just sayin'
Yes that is the bad thing now. I know about the other simple ways to create phthalic acid, however I do not have vinyl gloves by which to extract
minute amounts of pthalic acid for large sums of money. I do not have xylene so I can't simple oxidize it to get the phthalic acid.
Eventually I will be able to access toluene from my school lab and I could possibly try to make xylene by methylating it, (even though I would rather
stay away from methylating agents), and then obtain phthalic acid by oxidation with KMnO4. Right now I just have access to a lot of PET
bottles and a good supply of conc. aqueous ammonia.
So okay, I know the theory is complicated and a lot of things won't necessarily go off as planned, but the information gained from the reaction will
be good in the planning of other future reactions, even if it doesn't work. If it does work the reaction won't just be limited to the production of
carboxylic acids. The trihalomethyl-arene intermediate can be reacted with nucleophiles to produce a wide range of chemicals; that is in theory at
least.
Addressing a few comments:-
@smaerd- I was thinking more of fusing the iodo-phthalic acid with alkali to produce the hydroxylated phthalate salt. The removal of the phenol
functionalty was not with acid, which is symbolized by H+ or H3O+, but with reducing agents, symbolized by [H]. The
"Li-O-ligand", as you would have seen if you had read the earlier posts in the thread, is a bulky organic lithium alkoxide which is used as a
sterically hindered base.
Please remember that this is not the actual goal of the reaction, that is, to say produce phthalic acid. The aim is to test whether or not this
reaction is indeed feasible as I had outlined in the very topic of the thread. The only reason that I use a poly(ethylene terephthalate) derivative is
that I can produce large quantities of the compound easily and because if phthalic acid can be made I can verify it utilizing its derivative, luminol.
Thank You......
"Discoveries are not made by idly sitting around and hoping something interesting might happen; they are made
by getting out there and doing something to push the results and odds in your favour." "Chemistry always works... just not always in the way you
want."
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
You could always order some ortho-xylene or go to a hard-ware store, though I'm pretty sure thats a mix of isomers.
Well I figured you didn't mean hydrogen such as a hydrogenation reaction as those would reduce your carboxylic acid functionality, surely? Do you have
a reference for this lithium ligand reaction I'm pretty confused as to how a lithium alkoxide puts a hydroxyl on an aromatic ring?
Seriously I would scrap your whole schematic. I'm not saying it to be mean, but let's be honest there are serious flaws in it, and if you want to
carry it out it doesn't seem like you really know what you're doing or have performed these reactions before. If you can't get xylene how on earth do
you plan on getting things like NaNO2 for the sandmeyers, or Lithium metal, nitric acid, Hydrogen gas, or Pd/C, etc. I understand you've done a lot of
research here and invested a lot of time but this doesn't look like the way to get what you want, not even from what you want.
"that I can produce large quantities of the compound easily" Where's the easy part? I see a 13 step convoluted synthesis in place of something that
can be made in one step from a commonly available and cheap solvent with one commonly found reagent.
By methylation of toluene I'm sure you mean friedel crafts alkylation the electrophilic aromatic substitution reaction. In this case you would want to
do acylation as surely over-alkylation will occur.
I guess if you choose not to listen please be careful doing whatever it is that you are doing.
[Edited on 5-11-2012 by smaerd]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I think your proposed reaction might work if you use chloroform instead. But then to transform the -CCl3 group into a carboxyl group, you would need
to leave it for several months to react with NaOH solution, perhaps with a CuI catalyst.
While refluxing it would speed the reaction up to only 4 hours, it would also lead to decarboxylation, and you would just get nitrobenzene.
Another idea to speed up the reaction might be to use something like sodium methoxide, and then hydrolyse off the orthoesters.
[Edited on 6-11-2012 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by AndersHoveland  | I think your proposed reaction might work if you use chloroform instead. But then to transform the -CCl3 group into a carboxyl group, you would need
to leave it for several months to react with NaOH solution, perhaps with a CuI catalyst.
While refluxing it would speed the reaction up to only 4 hours, it would also lead to decarboxylation, and you would just get nitrobenzene.
Another idea to speed up the reaction might be to use something like sodium methoxide, and then hydrolyse off the orthoesters.
[Edited on 6-11-2012 by AndersHoveland] |
One would be most wise to always read the title under your name.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Update #1
I have a bit of good news. The lab technician has agreed to give me a decent quantity of toluene, that I can use for research (and 30ml of benzene).
So instead of synthesizing deactivated halo-arene compounds starting with poly(ethylene terephthalate), I'll be able to synthesize similar deactivated
aromatic halides compounds using toluene.
@smaerd - I might not be able to access everything I want, but if I can't buy it or extract it, I will make it.
I can produce the nitrite from the carbon reduction of potassium nitrate (which I can simply buy from pharmacies), then further purification to remove
oxide and hydroxide contamination. I can extract the lithium from energizer ultimate lithium batteries, with ease and then rinse the Li with some
toluene to remove the electrolyte. I already have some nitric acid that I got a good while ago. Remember I did not specify what type of reduction I
was going to use and I was not thinking of using palladium, carbon or platinum. But I was thinking of using nascent hydrogen generated in situ. But if
on the topic of reduction aiding metals, I can get nickel from energizer nickel metal hydride batteries as well.
Below are three reactions dealing with the generation of deactivated halo-arene compounds and their subsequent dehalogenation\carboxylation to
carboxylic acids.
Well, pertaining to the reaction scheme I think the first might be workable but the second and third might deviate due to steric effects however; as I
said this thread is for testing the reaction's possibility.
So since I got some toluene ( YAY!!!  ) I'm going to first isolate some bromine
for the bromination of the toluene to 2-bromotoluene and the reaction scheme should just fall into place. ) I'm going to first isolate some bromine
for the bromination of the toluene to 2-bromotoluene and the reaction scheme should just fall into place.
Truly, what really interests me about this reaction is that if it works a trihalomethyl-arene will result initially. Using benzotrichloride as a
suitable chemical analogue, this resulting trihalomethyl-arene will be a relatively reactive chemical intermediate, capable of hydrolysis to the
carboxylic acid. However, if other nucleophiles are utilized a wide range of chemicals might result, depending on nucleophile, and reaction conditions
etc...
Below is a proposed example of the compounds formed due to rxn of the trihalomethyl-arene with other nucleophiles.
@AndersHoveland - I think you might need to recheck whatever erroneous sources you got that reaction reference from. No offence but honestly I think
that would be best. I don't think decarboxylation can result in aqueous solution. I think you have that confused with the dry distillation of
carboxylates with calcium oxide or sodium hydroxide similar to the members publication http://www.sciencemadness.org/member_publications/benzene_pr... . The reaction described with the methoxide would most likely just result in the
conversion of the haloform to methyl orthoesters or eventually to formic acid if water is present as well.
Thank You......
"Discoveries are not made by idly sitting around and hoping something interesting might happen; they are made
by getting out there and doing something to push the results and odds in your favour." "Chemistry always works... just not always in the way you
want."
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Could always do something like this if you have toluene and want to do a bit of leg work and do some classic organic reactions.
Toluene -nitration-> Ortho-nitrotoluene -reduction-> ortho-amino-toluene -sandmeyer-> ortho-bromo-toluene -grignard with CO2->
ortho-methyl-benzoic acid -oxidation-> pthalic acid.
I think that would work and would be a fun project. Or maybe,
toluene -friedel crafts acylation with acetyl chloride-> ketone intermediate -clemmenson reduction-> 1-ethyl,2-methyl,benzene -oxidation->
target compound.
Those are just two ways I can think of reaching pthalic anhydride from toluene without doing anything exotic. Don't hold me too my iupac naming but
the reactions I'm talking about should be easy to follow.
----------------------
I'm really confused about your latest reaction mechanisms. I do not understand what they have to do with pthalic acid? Or is this thread just where
you ask people about reactions you've drawn up?
---------------------
I still would like to know what reaction that lithium alkoxide is, or a referance for it. Typically when a metal alkoxide reacts with a halo-arene it
forms a phenyl ether, and not a carboxylic acid. That's why I'm curious about it...
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Update #2 ...... Understanding
@smaerd - I keep insisting especially to you that the thread is not primarily about making phthalic acid. The primary reason for the thread is to
create a method by which deactivated halogenoarenes can be dehalogenated and carboxylated. Basically swapping a halogen atom for a carboxyl group on a
deactivated aromatic molecule; and it wouldn't be bad to synthesize other compounds from the intermediates as well  . I am asking persons opinions on the reactions I have worked out so I can adjust them
so they have the best chance of working. . I am asking persons opinions on the reactions I have worked out so I can adjust them
so they have the best chance of working.
I pretty much think I had covered the reaction of the alkoxide and it's importance to the entire reaction, but I will elaborate.
According to my research, when chloroform is reacted with a strong base, a trichloromethyl anion results. However this molecule will easily undergo
alpha elimination to produce a reactive chemical intermediate called dichlorocarbene. This intermediate is what is responsible for the formation of
aldehydes from activated aromatic compounds (Reimer-Tiemann reaction), due to the ring's rich electron density. However, since I am working with
deactivated aromatic compounds this would not work since the aromatic ring has less electron density. So even if dichlorocarbene was produced, a
carbonyl group would not have been added, and I would not be able to oxidize it to a carboxylic acid.
So I had to devise my own method. It incorporates nucleophilic aromatic substitution, the Halex Reaction (Finkelstein Reaction), the Reimer-Tiemann
reaction and effects such as the mesomeric effect and polar effects (electron withdrawing and donating effects).
Firstly, I researched chemical analogues to the trichloromethyl anion. Since treating chloroform with a strong base results in the formation of the
trichloromethyl anion then bromoform reacted with strong base should result in the formation of the tribromomethyl anion. This is where the lithium
alkoxide comes in, this will function as the base. A solution of the alkoxide (in an organic aprotic solvent) will be mixed with the bromofrom to
create a solution of the alcohol (due to deprotonation of the bromoform) and an intermediate lithium salt.
Now comes the nucleophilic aromatic substitution:-
After forming the intermediate lithium salt the deactivated haloarene compound is then added. Hopefully, what would occur on a molecular level is that
the nucleophilic tribromomethyl anion would attack the carbon attached to the halogen atom. Eventually what would occur is that the halogen being the
better leaving group, (due to formation of an adduct with a compound added beforehand, which helps weaken the halogen-carbon bond), would be displaced
from the molecule as halide and the halide salt would precipitate out of the organic mixture (halex reaction scheme). The precipitation would drive
the reaction forward due to Le Chatlier's principle. The deactivated tribromomethylarene, which is similar to benzotrichloride in chemistry, would
then show a great deal of increased reactivity; such in that it could be easily hydrolyzed to the carboxylic acid derivative or reacted with other
nucleophiles to result in the formation of other compounds.
I hope this clears up any misconceptions (a bit of the details have been omitted).
Thank You......
"Discoveries are not made by idly sitting around and hoping something interesting might happen; they are made
by getting out there and doing something to push the results and odds in your favour." "Chemistry always works... just not always in the way you
want."
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I will not (yet) partake into this discussion, but will only give some (otherwise obvious) suggestions on how to improve it.
Here you have a good starting point for a proper introduction in the topic. For example: What exactly is wrong with the already existing reactions or
strategies for the ArylX => ArylCOOH transformations? There are already several ways to do that and by ignoring and avoiding to review them, you
make your proposal look obsolete which is certainly not a good way to attract other members into the discussion.
Additionally, you keep on presenting ideas that go contrary to the current understanding of reaction mechanisms or have no reference. That is another
detracting factor, because people who would like to (constructively) partake the discussion might get the impression that their replies would be in
vain. I suggest you to change style. If you can't change, then at least limit your ideas to what can be referenced. This way there will at least be
something to discuss about. Discussing non-existing evidence about non-existing experiments is futile (chemistry is not metaphysics!). Chemists do not
discuss the mechanisms of non-existing reactions - if they have an idea, they do the experiment. Only if the experiment is successful, are further
experiments that could provide mechanistic evidence planned. This is part of the scientific method.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
|