| Pages:
1
2 |
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Benzotriazole and derivatives
As benzotriazole is now a widely available and relatively cheap chemical used in the domestic environment as a corrosion inhibitor in circulating
water heating systems I thought it would be interesting to see what chemicals could be prepared from it. There are three possible modes of attach that
I can see, the first is the imino hydrogen on the triazole ring, the second is direct electrophilic substitution of the benzene ring and the third and
potentially most useful is the cleavage of the benzene ring to give a substituted 1,2,3 triazole derivative (see The Chemistry of Heterocycles by T.
Eicher and S. Hauptmann).
The imino hydrogen tautomerically distributed between the 1 and 2 positions so alkalating agents tend to give a mixture of 1 and 2 substituted product
though 1 generally predominates. Other reactions tend to attack the 1 position preferentially. The imino group can be halogenated to give 1-halo
derivatives which in common with other haloimine (ie N-bromosuccinamide and TCC) acts as halogenating agent.
The second reaction seems fairly limited as the benzene ring is not particular reactive and so requires fairly aggressive conditions to bring about
substitution, however it can be directly nitrated in the 4 position and concentrated hydrochloric acid an oxidizing agent such as nitric acid give
4,5,6,7 tetrachlorobenzotriazole.
The most interesting reaction, however, is the oxidation with potassium permanganate which cleaves the benzene ring and generates
1,2,3-triazole-4,5-dicarboxylic acid. This compound is potentially the starting material for many other compounds, it is likely to lose a carboxylic
acid group fairly readily to give the 4-monocarboxylic acid. Both the mono- and di- carboxylic acid should esterify by the Fischer process in
methanol and the esters can be converted to the amide and thence to the nitrile. Though by analogy with pyrazine-2,3-dicarbonylamide the dehydration
to a vicinal dinitrile requires aggressive conditions (thionyl chloride) and yields are poor. There may also be a possibility of using Hoffman’s
rearrangement of the amide to access the mono-amine though again by analogy with the pyrazine equivalent it will probably not work with diamide (Paul
E. Spoerri & A. Erikson JACS 1938 p400).
The 4,5-dicyano-1,2,3-triazole is an energetic material and acts as a monobasic acid, it is usually prepared from the very inaccessible
diaminomaleonitrile (aka hydrogen cyanide tetramer C4N4H4) and nitrous acid.
So here’s the results of my first attempts at oxidizing benzotriazole to the triazole dicarboxylic acid.
My 1,2,3-Triazole-4,5-dicarboxylic acid Preparation
The reaction is assumed to proceed in accordance with the following equation:
6KMnO4 + C6H5N3 -> C4HN3O4K2 + 2K2CO3 + 2H2O + 6MnO2
160g + 20g = 39g + 46.4g + 6g + 87.6g
And:
C4HN3O4K2 + 2K2CO3 + 6HCl -> C4H3N3O4 + 6KCl + 2H2O + 2CO2
39g + 46.2g + 36.6g = 26.3g + 74.8g
20g of technical grade benzotriazole were dispersed into 400ml of water at 50°C and then 150g of finely powdered potassium permanganate added in very
small portions of about 1g at a time to the mechanically stirred mixture in a 2L beaker.
The initial reaction generates considerable heat and the purple colour of the permanganate is rapidly replaced by a brown precipitate of manganese
oxide. Initially there is considerable foaming and the stirrer must be run at a fairly high speed to help break up the foam. It also helps to rinse
down the sides of the beaker every so often with a wash bottle. If the temperature exceeds 75°C, which it did several times, add a little crushed ice
and suspend addition of the permanganate until the reaction mixture has cooled a little. The addition took about 2 hours but after the first 30
minutes the foaming ceased.
Once addition of the permanganate was complete the dark brown slurry was stirred until almost cold, no residual permanganate was left so the slurry
was filtered at the pump and rinsed with about 50ml of cold water. The manganese dioxide cake was preserved and dried for chlorine production.
The light brown filtrate, about 470ml, was boiled with 2g of decolourizing carbon and filtered again while still hot to give a clear straw coloured
solution. This was evaporated down on a water bath to about 80ml by which time it had acquired a dark brown colour so was treated with a further 1g of
decolourizing carbon and filtered hot. On cooling the light brown solution deposited long, almost colourles blades. The solution was warmed until the
crystals dissolved and transferred to a 250ml beaker and acidified with 30% hydrochloric acid until the solution was distinctly acid to Congo Red
paper. There was much foaming at first as the potassium carbonate was decomposed and then the solution because much paler and deposited a flock-like
precipitate. The small amount of flock like precipitate was filtered off and the pale straw coloured solution reduced to about 100ml on the water bath
and cooled overnight. Masses of minute, pale straw coloured prisms formed which were recovered at the pump and sucked as dry as possible. They are
rather soluble so no attempt was made to wash then on the filter; the yield of crude dicarboxylic acid was 8.63g of pale straw coloured crystals when
dry. These crystals melt when heat on a spatula and burn away leaving a trace of white soluble residue (KCl).
The filtrate was evaporated do to roughly half its original volume (to about 50ml) and allowed to cool. A heavy almost white granular crystalline
precipitate formed that looked very different from the first crop of crystals. The crystals were practically infusible on a spatula and decrepitate
badly. Chemical test reveal that it is mostly KCl.
The first crop of crystals has not yet been re-crystallized.
I intend to try this oxidation again but using a lower temperature and a slight excess of potassium permanganate to see if I can improve the yield. I
also intend to see if I can esterify the acid.
Has anyone else experimented with this material
 The reaction mixture after addition of 100g KMnO4 and some ice The reaction mixture after addition of 100g KMnO4 and some ice
 After filtration and evaporating down to 80ml prior to 2nd Norit treatment After filtration and evaporating down to 80ml prior to 2nd Norit treatment
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Thank you very much for sharing your experience and please post about the recrystallization, characterization and yields, when you finish. You might
as well write a report in the Prepublication section.
The product is indeed a very interesting starting material for further work from the amateur viewpoint, as most derivatives will be crystalline
solids.
Is the procedure described in The Chemistry of Heterocycles or does this lead to another reference? I can only find Chinese patents and
articles for this oxidation of benzotriazole with KMnO4.
According to EP0847993 (Example VI), the oxidation with HNO3 in the presence of 3.8 mol% Fe(NO3)3.9H2O,
followed by H2O2 after 16 h at 50 °C, gives a 81% conversion to 1,2,3-triazole-4,5-dicarboxylic acid. The isolation procedure
is not described, but could be cleaner and with good yields, considering there is no need to mess with MnO2 waste. The method is claimed to
work also on benzimidazoles, (iso)quinolines and related benzoazines and benzoazoles.
Benzotriazole used to be easily available as a photography chemical years ago. Since the digital photography decimated the suppliers, it is good to
know it become again available through other suppliers.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Just out of curiosity, what product is benzotriazole available from? This is pretty interesting, as I've been looking for a source for a while. I have
a small amount of o-phenylenediamine which can be diazotized to form benzotriazole. However, I need quite a lot of it for a reaction and I have not
even close to enough phenylenediamine.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Nicodem
Thank you for your comments. My company send me all over the place so I often end up a long way from my lab (as at present) but when I get home in the
in mid November I will recrystallise it and run a M.p. determination. I must admit the use of K permanganate as the oxidant is my own idea but I have
used this process to prepare anthraquinone 1,2 dicarboxylic acid before and I have a very old book on anthraquinone and its derivatives which
describes the use of K permanganate. Permanganate seems to be more selective than dichromate and the work up is much simpler and my experience with
nitric acid is that it usually requires higher temperatures and therefore a pressure vessel. So I haven't tried Nitric acid or peroxide but I will
definitely give this one a go as the patent claims only 50 C. I didn't run a search on oxidations reactions of this type and the Chemistry of
Heterocycles is rather poorly referenced, it just states that oxidation of benzotriazole gives the triazole dicarboxylic acid. I do have a reference
to a similar oxidation but I can't find it jusat present but it recommends keeping the temperature below 40 C during permanganate oxidation so when I
re-run the experiment I'll use external cooling and more crushed ice.
I like the sound of the nitric acid/Fe salt/H2O2 oxidation. Thank you for this lead, I downloaded the patent and I'll check it out and get back in
November!
Benzotriazole is available on ebay (.co.uk version at least) at 65 pounds stirling for 5kg! I bought 1kg from my local industrial plumbing supplier
for 28 pounds (about $45) under the name "Cobratec" or "Cobratec 99" but do a search on the net and I bet its available under different names
elsewhere. "Cobratec TT" is the 5-methyl-benzotriazole and yields the same product on oxidation but requires a lot more oxidant.
Has anyone experience of esterifying this type of diacid? For various reasons (I have reference of Pyrazine carboxylic acid ester) I expect the
diesters to lose one carboxylic acid group at elevated temperatures so I was thinking of trying to esterify it at lower temperatires using anhydrous
methanol and HCl gas.
By the way I recrystallised the Cobratec from water before use but the product is a white woolly mass of fibres. I know from past experience that
benzene is better because it give a free flowing granular crystalline product. I no longer have access to benzene but toluene might be OK I'll try it.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have found the attached reference that reviews the chemsitry of 1,2,3 triazoles and it contains several descriptions of triazoles preparations by
potassium permanganate oxidation of a suitable precursor.
Well I can't attach it because it over 2MB so if anyone want to look at it contact me by U2U
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
We can get the paper by ourself if we only have the reference, so how about posting the reference? Besides, it is good practice to cite the reference
even if you attach the full text article as a file. This way it makes it useful for those who ever bother to UTFSE. When a file is too big for being
attached, you can always upload it to the forum FTP and post here the link.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Sorry about that you're right. Here is the reference;
The Chemistry of the Vicinal Triazole; Frederic R. Benson and Walter L. Saville; 1948 p1-68 unfortunately I can't see what journal its from though for
several reasons I think it might be Chemical Reviews
Vicinal triazoles if the old term for 1,2,3 triazoles
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
What about nitrating the benzotriazole, then forming a lead or copper salt?
The lead salt of dinitrobenzotriazole might have good properties as a detonator.
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Eicher and Hauptmann only mention the mono nitro compound and state that the main product of direct nitration is the 4 nitro compound. It may be
possible to force a second nitro grounp onto the benzene ring, most likely in the 6 position. Alternatively it may be possible to obtain this material
via picric acid.
Picric acid + PCl5 => Picryl chloride
Picryl chloride + NH3 => 2,4,6 trinitroaniline
2,4,6 TNA + NaS => 4,6 dinitro 1,2 phenylene diamine (by analogy with formation of picramic acid)
2,4 DN oPD + NaNO2 + HCl => 4,6 dinitrobenzotriazole
Benzotriaziole is acidic, in fact this was how I first came across it at Univ where it was used (at that time) as a gravimetric reagent for silver. I
never thought to test the silver salt to see if it was energetic but the 4,6 dinitrobenzotriazole should be a slightly stronger acid and potentially
moderately energetic.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
The story continues:
Having had further time in the laboratory I have now recrystallised the 8.6g of crude straw coloured triazole dicarboxylic acid. I found that the
material can be recrystallized with good recovery from water using 5ml per g. 8.63g were recrystallized from 40ml of water with the addition of 0.1g
of decolourising carbon and filtering through a preheated buchner funnel followed by cooling, final in the fridge overnight. 7.48g of pure white
product were recovered after drying.
 Straw coloured triazole dicarboxylic acid 1st recrystallisation Straw coloured triazole dicarboxylic acid 1st recrystallisation
 Pure white acid after 2nd recrystallization Pure white acid after 2nd recrystallization
The liquor left from the recrystallization was not discarded but was kept and with be worked up with the solution that resulted from boiling the Mn
oxide cake with 300ml of fresh water.
Nicodem referred to a patent that desribed the oxidation of various heterocyclics with hydrogen peroxide, nitric acid and ferric nitrate as a
catalyst. I downloaded the patent and decided to give it a try. I used the technique described in the patent almost to the letter except that I used
ten times the quantity and added the nitric acid in 2 goes uncase the reaction turned violent; it didn't. I will post the details of the experiment
later but basically after neutralizing most of the nitric acid a mass of yellow crystals formed. When recrystallized from water the filter cake
consisted of a mixture of two products. An almost white fibrous material which appears to consist entirely of unreacted benzotriazole and a second
compound as blocky yellow crystals in the ratio of about 2:1. I have been unable to seperate them by recrystallization but when recrystallized from
water in the presence of a little EDTA the crystals of both are practically coourless so the colour is probably due to iron. I will continue my
investigations of this reaction and the product later this month.
  Left; solution after oxidation for 18hrs & Right; crystallization after neutralizing Left; solution after oxidation for 18hrs & Right; crystallization after neutralizing
  Left; the raw filter cake & Right; recrystallized material filter cake, the 2 phases clearly visible Left; the raw filter cake & Right; recrystallized material filter cake, the 2 phases clearly visible
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I decided to try the hydrogen peroxide oxidation of benzotriazole again but this time using less and stronger nitric acid. The very slow addition of
hydrogen peroxide means that its concentration is always very low. So I mixed the benzotriazole (quantities as above) with the ferric nitrate and
placed them in the 1 litre 3 neck flask in a water bath with a reflux condensor and thermometer and a dropping funnel to add the H2O2. I added 10ml of
50% nitric acid made by diluting conc (70%) nitric with 35% H2O2, nothing happened so I added a further 10ml. Just as I had completed the second
addition a reaction commenced and the mixture began to froth; as soon as I turned on the magentic stirrer the reaction suddenly became exceeding
vigorous and filled the flask with foam and brown fumes. Curiously the gas escaping from the top of the reflux condenser was only slightly coloured
with only a slight smell of NO2. When the reaction had subsided I placed the 35% in the dropping funnel but did not add the remainder of the nitric
acid/H2O2 mixture.
Even small drops of H2O2 caused a vigorous reaction, often after a short induction period, but eventually I got the addition rate to a level where the
mixture reacted vigorously and continuously. No external heat was require from the water bath and the reaction temperature settled at about 70 C. The
addition was over in less than 2 hours. Towards the end the temperature began to fall and after complete additon the water bath was turned on and
raised to 55 C for and hour to complete the reaction.
A small amount of sodium hydroxide (16ml of 40% solution) were added to neutralise most of the nitric acid and the solution poured into a 250ml beaker
to cool. Masses of yellow needles formed as before but in a smaller quantity. After 24hours at lab temperature the solution was stirred to slurry the
crystals and filtered at the pump and washed with a little cold deionised water. The cake was dissolved in the minimum quantity of deionised water
heated to 70 C (about 150ml) and the clear solution slowly cooled. After a few hours only blocky yellow crystals had formed and even after placing in
the refrigerator overnight only a trace of white fibrous material crystallised. The recovery was rather lower than the first experiment but the
conversion was more complete; a second recrystallisation gave a pure straw yellow beautifully crystalline compound that is entirely different from the
permanganate oxidation product. It is not particularly acidic and does not dissolve more readily in alkalis than in water. A little material was heat
in a small porcelian crucible and it decomposed at its melting point but was not the least energetic and the residue contained only traces of iron
suggesting that the colour is inherent in the compound. Further work will be required to identify the product of this oxidation but it is clearly not
1,2,3 triazole 4,5 dicarboxylic acid and I now doubt the validity of the patent.
I intend to produce some of the triazole dicarboxylic acid via a differnt route, that is by hydrolysis of the dicyanide which is available via the
commerically available (but expensive) dicyanomaleonitrile. I will also re-run the permanganate oxidation and work up the Mn oxide residue.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Not really necessary. Aniline can be acetylated to protect the amine group, then nitrated, and finally hydrolyzed with ammonia to remove the acetyl
group. Acetic anhydride is safer and more readily available than PCl5, the procedure would be much simpler.
Quote: Originally posted by Boffis  |
4,6 dinitro 1,2 phenylene diamine (by analogy with formation of picramic acid)
2,4 DN oPD + NaNO2 + HCl => 4,6 dinitrobenzotriazole
|
Actually, as was discussed in the "3 - Nitroanilinediazonium perchlorate and derivatives" thread, the diazotization of such a compound, where
a nitro group is in an ortho- or para- position relative to the amine, is more difficult, since the nitro groups will be electron-withdrawing from the
amine through the aromatic ring. Nitrous acid by itself is unable to oxidize the amine group in this situation, but nitrosyl ions, NO+,
ions can. Basically what this means is that hydrochloric acid is not going to be strong enough. One would have to use 70% conc. sulfuric acid with the
nitrite for the diazotization to proceed.
Quote: Originally posted by Boffis  | | I decided to try the hydrogen peroxide oxidation of benzotriazole again but this time using less and stronger nitric acid. I will also re-run the
permanganate oxidation. |
It may be possible that some of the benzotriazole is being oxidized to the N-oxide. For example, the nitration of 4-nitro-1,2,3-triazole, which
proceeds quite readily, results not in the di-nitro derivative, but in 4-nitro-1,2,3-triazole-3N-oxide. I have no doubt that
persulfate would also oxidize benzotriazole to the N-oxide, although perhaps other degredation products would also be formed.
[Edited on 10-2-2013 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Anders Hoveland
"Not really necessary. Aniline can be acetylated to protect the amine group, then nitrated, and finally hydrolyzed with ammonia to remove the acetyl
group. Acetic anhydride is safer and more readily available than PCl5, the procedure would be much simpler."
I can buy PCl5 much more easily than acetic anhydride and in large quantities. The nitration of phenol and certain phenolic derivatives to picric acid
is well trodden ground and I can get excellant yields. I bought some acetanilide off ebay about a year ago and tried nitration and polynitration. I am
not sure I got the trinitroacetanilide to start with but when I tried to hydrolyse it I sure as hell didn't get trinitroaniline. According to COPAE it
is prepared by direct nitration of aniline in glacial acetic acid but I haven't tried this. I have made picryl chloride several time over the years
though I haven't tried to convert it to trinitroaniline.
"Basically what this means is that hydrochloric acid is not going to be strong enough. One would have to use 70% conc. sulfuric acid with the nitrite
for the diazotization to proceed."
Probably true but I was merely speculating. On reflection its probably academic since further reading suggests that the reaction of alkalis and alkali
sulphides tend to be violent and therefore simply yield a lot of decomposition products (see picramide in COPAE).
"It may be possible that some of the benzotriazole is being oxidized to the N-oxide. For example, the nitration of 4-nitro-1,2,3-triazole, which
proceeds quite readily, results not in the di-nitro derivative, but in 4-nitro-1,2,3-triazole-3N-oxide."
Yes this thought had occurred to me too and since N-oxides often nitrate more readily than the parent heterocyclic this seem a real possibility,
however, the nitro group will almost certainly be on benzene ring which nitrates more easily anyway. If the nitro group enters first then I may have
4-nitrobenzotriazole ?N-oxide.
The product of H2O2-HNO3 oxidation is a well defined, beautifully crystalline, compound that I have yet to investigate throughly but a few properties
are already evident. It melts with decomposition but is not the least bit energetic and it is less acidic than the parent compound (the simple nitro
compound you would expect to be at least as acidic). When dissolved in hot NaOH it is decomposed giving a clear solution from which nothoing can be
precipitated on acidification.
Would you expect the imino hydrogen to be less acidic on an N-oxide?
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have now had time to do some further research into the synthesis of 1,2,3 tirazole-4,5-dicarboxylic acid from benzotriazole by oxidation.
Revisiting the potassium permanganate procedure I have discovered that dissolving the benzotriazole in a molar equivalence of potassium hydroxide
before the addition of the potassium permanganate results in smooth oxidation, no frothing and a much cleaner filtrate. In fact the filtrate is a pale
golden colour after filtration and turns lemon yellow on acidification and the filtered raw product is almost white. The pure acid was obtained in
69.5% yield and further working up of the Mn oxide cake and the recrystallisation liquor should allow this to be raised to 72-73% recovery of an
excellant quality pure white triazole derivative. To get the high recovery careful temperature control is essential as is the repeated work-up of the
brown manganese oxide filter cake since each sucessive washing recovers about one quarter of the previous filtration. With 40g of benzotriazole the
yield of acid from each of three washings was as follows:
1st filtration 33.66g of crude product
2nd filtration 8.83g
3rd filtration 2.73g
It is therefore anticipatated that a further washing would only yield about another 0.7g of triazole 4,5 dicarboxylic acid and it was felt that this
was not worth the effort. Using a larger volume of wash water would slightly increase the yield without a forth washing.
I will probably post the full write up in the publication section later.
I also continued my investigation of the Fe/H2O2/nitric acid oxidation procedure, The coarse yellow blocky crystals appear to be an iron salt from
which it is difficult to remove the iron (a complex rather than simple salt). I eventually found that recrystallisation from disodium EDTA solution
removes the iron but the product is still faintly straw coloured. Its identity is still not known.
@ Anders I have found out that picramide (2,4,6 trinitroaniline) can be prepared from picryl chloride and a source of ammonia but I also discovered a
paper that describes the preparation of picramide from picric acid and excess urea. The result being picramide and cyanuric acid.
See; Preparation of Picramide, Canadian J Research, Spencer & Wright, 1946, p204
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@Boffis,
Very nice work!
Triazole 4,5-bicarboxylic acid is a way to get to 4,5-diamino-triazole.
I wonder if diazotation of this would lead to two triazole penta-rings scotched by the C=C.
The structure must be planar and biacidic (HN3C2N3H).
About the nitration of benzotriazole nitration can also happen on the N
See http://www.uni-tuebingen.de/ziegler/papers/sc_2010_3046.pdf
There is an evident structure parallelism between dinitrobenzotriazole and Diazadinitrophenol.
So DNBT might be sensitive.
Except DNBT is acidic and might form metalic primaries.
[Edited on 29-1-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@PHILOU Zrealone, thank you! That is an interesting paper and it looks do-able as far as the initial nitration is concerned though the further
reactions seem to require sodium hydride which is not easily obtained.
I must apologies for letting this project lapse for so long unfortunately I work overseas a great deal at present and so don't get much time to pursue
my hobby. I have however, now obtained a good melting point instrument and lots of glass capillaries so I hope to make the future work more
quantitative. I have also obtained some of the 5-methyl substituted benzotriazole (Cabrotec T) in the hope that, as with toluene, it is easier to
nitrate on the benzene ring than its parent. I have also splashed out and bought some diaminomaleonitrile (C4H4N4) to allow me to get to the triazole
derivatives "from the other end" using well established published procedures to provide material for comparison.
My next move will be an attempt to esterify the acid and to attempt partial decarboxylation to give me di and mono-esters. From these I hope to be
able to get to at least the mono amine and nitrile and hopefully the 4,5 di-substituted derivative too, though I am less hopeful. If I can get to the
diamine I'll give the fused bitriazole preparation a shot too. I am also planning to try to turn the nitriles into ketone with methylmagnesium iodide
which should give me access to some interesting ligands.
I still haven't made any progress with the mysterious straw yellow material from the H2O2 oxidation but perhaps a mp determination will shed some
light on it. Watch this space about the middle of the year!
One last thought that I am working on that someone out there may be able to comment on (Nicodem!). I have acquired some 2-nitro-1,4-diaminobenzene
dihydrochloride (used in hair dyes), its rather old but looks reasonably good (brownish crystals). If I reduce the nitro group to an amine to give me
1,2,4 triaminobenzene do you think I will be able to react it with one molar equivalence of sodium nitrite+HCl to give 5-aminobenzotriazole? In other
word are all three amino group equally reactive towards nitrous acid or will the ortho pair react preferentially?
There is also an organic synth preparation of 4-nitro-1,2-diaminobenzene from 2,4 dinitroaniline which looks like a possible route to
5-nitrobenzotriazole.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  | Alternatively it may be possible to obtain this material via picric acid.
Picric acid + PCl5 => Picryl chloride
Picryl chloride + NH3 => 2,4,6 trinitroaniline
2,4,6 TNA + NaS => 4,6 dinitro 1,2 phenylene diamine (by analogy with formation of picramic acid)
2,4 DN oPD + NaNO2 + HCl => 4,6 dinitrobenzotriazole
|
@ Anders
I have recently found a series of papers that cover the selective reduction I guessed at above. The most relevant paper is:
Norton & Elliott, Berichte v11, p327 (1878)
This paper describes the reduction of picramide (2,4,6-trinitroaniline) to 4,6-dinitro-1,2-phenylenediamine.
The only thing I can find out about 4,6-dinitrobenzotriazole is that it is available commercially from Molport in the US ... at a price! >1000x the
price of gold!!! 2 milligrams for $32 (a bargain?)
However since the compound is available I suspect the diazo-cyclotization reaction is possible.
Also of interest in relation to my experiments with the H2O2-HNO3 oxidation of benzotriazole is that there is a fair amount of literature on
2-substituted-benzotriazole-N-oxides. They probably exist tautomerically with 1-hydroxybenzotriazole and are only stabilized by the replacement of the
H in the 2 position with a functional group.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Boffis  |
One last thought that I am working on that someone out there may be able to comment on (Nicodem!). I have acquired some 2-nitro-1,4-diaminobenzene
dihydrochloride (used in hair dyes), its rather old but looks reasonably good (brownish crystals). If I reduce the nitro group to an amine to give me
1,2,4 triaminobenzene do you think I will be able to react it with one molar equivalence of sodium nitrite+HCl to give 5-aminobenzotriazole? In other
word are all three amino group equally reactive towards nitrous acid or will the ortho pair react preferentially?
There is also an organic synth preparation of 4-nitro-1,2-diaminobenzene from 2,4 dinitroaniline which looks like a possible route to
5-nitrobenzotriazole.
|
Hi Boffis,
I think that in 1,2,4-triaminobenzene, the 3 NH2 are equivalent towards diazotation but the formation of the triazole ring is very fast.
In typical aromatic amine diazotation with excess aromatic amine, one usually get a side reaction of azo-coupling...that's why NaNO2 and HCl are used
in slight excess otherwise aniline diazotizes and react in para position of another aniline molecule.
Ar-NH2 + HONO --> Ar-N=N-OH + H2O
Ar-N=N-OH + Ar-NH2 --> Ar-N=N-Ar-NH2 + H2O
In the case of o-diaminobenzene, as you have observed, no coupling occurs between o-diaminobenzene and o-amino-benzodiazonium because the
intramolecular NH2 reacts very fast with the viccinal diazonium and cyclise into benzotriazole before it has a chance to react extramolecularly.
You will thus have diazotation in the 3 positions 1, 2 and 4.
1°)Positions 1 and 2 are equivalent because the derivated benzotriazoles are resonant.
-C-N=N-NH-C - <==> -C-NH-N=N-C -
So actually 4-amino-benzotriazole is also 5-amino-triazole.
In the case you work stoechiometrically, you would end up with 2/3 of 4-amino-benzotriazole.
2°)Position 4 may also be diazotised, if you work stoechiometrically, the resulting 3,4-diamino-benzodiazonium will react with another identical
molecule in position 6 (only free para position to the NH2 in position 3, position 4 is oposed to the diazonium).
The resulting molecule can be polymeric in nature:
-Ar(NH2)2(-N=N)-)n
but it can also be dimeric
(H2N)2Ar(-N=N-)2Ar(NH2)2
Indeed the two ortho diazo bridges can interact to form a tetraaminodibenzotetraazapentalene related to tetranitrodibenzotetraazapentalene
(TACOT). Further diazotation would then add two triazole rings at the extremities because each end of the molecule displays ortho-diamino groups.
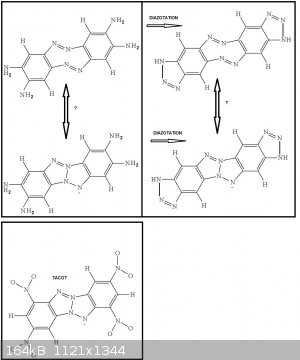
or trimeric
((H2N)2Ar(*)(-N=N-)Ar(NH2)2(-N=N-)Ar(NH2)2(-N=N-)(*))
(the * binds together to cyclise the molecule)
In a triangular fashion with aromatic rings on each tops (with two ortho NH2 groups on each aromatic ring pointing where the triangle top points to)
and with diazo bridges as the sides of the triangle to join two aromatic rings 2 by 2.
Here again further diazotization will induce triazole ring extension to the ends of the triangle.
3°)If exces HNO2 is used then you will end up with 4-diazonium-benzotriazole
[Edited on 3-8-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@ Philou Zrealone
Your post raises some fascinating possibilities that I was not aware of or had not thought about; I have now had time to think about this a good deal
and done a fair bit of research too. I am currently far from home so no experiments are possible at present but when I return home I will try the
reduction of 2-nitro-phenylenediamine and then diazotizing it with a single molar equivalence of nitrous acid or perhaps a more (x2) and see what we
get! My thinking on the resultant mixture is that simple diazo linkages can be reduced back to amines, so the ortho diazo linked benzotriazole
compound should be reducible to 5,6 diamino-benzotriazole with something like sodium dithionite so allowing the recovery of some potentially useful
by-product, the problem may be separating the product efficiently. However, see my comments below about reaction rates of competing coupling
reactions.
Theory is one thing but some experimental data is required here so I will give this reaction a go when I have time at home. By the way the resulting
aminobenzotriazole would be the 5 isomer;
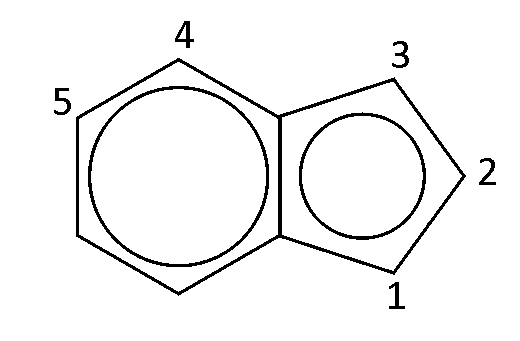
I have also discovered an interesting synthesis (2) of a fused quinoline-triazole-arsonic acid from the 1,2,4 triamine using toluenesulphonyl
substitution to protect one of the o-amines. This reaction was carried out in a single step, the arsonic acid entering the 4 position! This suggests
that the diazotization of the free o-amine group and its cyclotization to a triazole occurs rapidly with the cleaving of the tosyl group faster than
it can condense with the arsenite ions leaving only the 4-amino position to undergo Bart's arsonic acid synthesis. This suggests that it may be
possible to protect both amino groups in my 2-nitro-1,4-diaminobenzene with tosyl chloride, then reduce the nitro group with say H2S or ferrous ions
(avoiding strongly alkaline and acid conditions which might hydrolysis the tosylamine groups) to 1,4-ditosyl-1,2,4-triaminobenzene and then diazotize
the new amino group to give the 5-tosylaminobenzotriazole. Any comment on this idea? Since I have the materials I'll give this a shot too when I have
time.
An alternative has just occurred to me; Griess (6) described the coupling of diazotized sulphanilic acid with an o-diamine to give a triazole. Since
cyclotization reactions of this type are usually fast and the products stable it may be possible to react diazotised sulphanilic acid with 1,2,4
triaminobenzene in HCl solution to get mainly the 5-aminotriazole because the competing reaction, coupling to the 4-amino site to give an initial
aminoazo compound which will then probably rearrange to an o-amino-azo compound, is likely to be slow in an acid solution (think of the formation of
aminoazobenzene and its rearrangement to p-aminophenylazobenzene). The initial coupling requires weakly acid conditions and rearrangement occurs in
more acid condition because the initial compound dissociates into the diazonium compound and amine again and the second o-coupling reaction is much
slower so only competes in an acid solution. It may be possible to inhibit the 4 and 5 coupling reactions completely with sufficient acid. Furthermore
it may be than the diazo-cyclotization of the 1,2 diamine occurs so readily that it limits the scope for diazotization of the 4-position amine to a
minor by-product. I certainly feel that there is scope in this reaction to get a reasonable yield of a triazole.
While thinking about the preparation of other benzotriazoles I came across a reference to the synthesis of 1-Hydroxybenzotriazole via the condensation
of hydrazine with 2-nitrochlorobenzene, Sauron had discussed this reaction back in 2007 as a possible route to 2-nitrophenylhydrazine and thence to
1-hydroxybenzotriazole but it looks like the forcing condition required cause the hydrazine moiety to react with the nitro group give the hydroxy
triazole in a single step. This reaction is referred to in Eicher & Hauptmann (p207) but unfortunately the reference at the end of the paragraph
is to the preparation and use of simple hydroxy triazole derivatives (1) and I can't track down a reference for this reaction. However, this reaction
raises a whole series of possibilities e.g. could 2,4-dinitrochlorobenzene (of which I have plenty) be subject to a similar condensation to give
5(6)-nitro-1-hydroxybenzotriazole and 2-nitro-1,4-dichlorobenzene (available from p-dichlorobenzene + sulphuric acid + alkali nitrate) to give 5 or
6-chloro-1-hydroxybenzotriazole. I have found a reference to this reaction with 2,4-dinitrochloro-naphthalene with phenylhydrazine to give
2-phenyl-5-nitronaphtho(1,2)triazole-3-oxide so there is some merit in this idea (3) and other references to similar reactions of active o-halogens
with hydrazines have also been found; some examples results in a simple triazole while other in a mixture of a triazole plus a 1,2-bi-substituted
hydrazine. Nitro groups on the benzene ring can be reduced to amines and these active this ring to further substitution, these reactions were
extensively covered by Fries and Zincke ((7) many references).
Has anyone come across the reaction of 2-nitro-aromatic amines with hydrazine? The significance of such a reaction is its attack via the nitro group
and therefore overcome the problem of which amino group in 2-nitro-1,4-diaminobenzene will react, I vaguely remember seeing such a scheme once but I
might be mistaken. An internet search revealed only reduction to amine but in the presence of large amounts of Mg (the hydrazine seems superfluous to
me), I have found a reference to the reduction of 2-nitrobenzenediazonium compounds to the N-hydroxybenzotriazole with hydrazine; the use of sulphite
gives the hydrazine so conditions must be important (4).
@ Anders:- I have found a reference to the preparation of 4,6-dinitrobenzotriazole from picramide via 3,5-dinitro-1,2-diaminobenzene as I speculated
about above and it does work in HCl (5).
All of this, however, is moving away from the spirit of my original idea which was to explore the compounds that could be prepared from commercially
available benzotriazole and methylbenzotriazole through either substitution or degradation of the benzene ring. I haven't yet had time to follow up
the esterification of the acid or its partial decarboxylation followed by esterification to the monoacid ester. From these esters I intended to
attempt the preparation of the amides followed by either Hoffman's degradation to the amine or dehydration the nitrile.
I have also discovered another oxidative degradation of the benzene ring in benzotriazole via intensive chlorination and hydrolysis/oxidation to give
4,5 di-substituted triazoles with chlorinated or undersaturated substituents, there are many reference to this by Fries and Zincke in J.L. Annalen
Chem and Berichte, it will take some time to check these out and see which one are the interesting ones (7).
One curious thing is that I have not found a single reference to simple dimethyl or diethyl esters of the 4,5 dicarboxylic acid and only very few to
any ester at all; then mostly to N-substituted phenyltriazole carboxylic acids. If anyone has access to a chemistry specific search I would appreciate
any references you can find on their synthesis and properties.
@Philou: Do you have a reference to TACOT?
(1) Reference in; The Chemistry of Heterocycles 2nd ed - T. Eicher, S. Hauptmann:- M. Begtrup, P. Vedso, Acta Chem Scand, 1996, v50 p549; This refers
to the synthesis of N-Hydoxy-1,2,3-triazoles and 4 or 5 substituted analogues.
(2) Slater, R. H.; J Chem Soc; 1932, pp2196-7
(3) Mangini, A.; Atti accad. nazl. Lincei, Classe sci. fis. mat a nat. 25, 326-332 (1937) I have not been able to find this reference
(4) temporarily lost; to follow
(5) Nietzki, R. and Hagenbach, H.; Berichte. 30, pp544-545 (1897)
(6) Griess, P. Berichte; 1882; v15; p2199
(7) see refs in Benson F. R. & Savell, W. L.; Chemistry of Vicinal Triazoles; Chemical Review, 1950 p1-68
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Hi Boffis,
I have made two mistakes in my last reply...
"So actually 4-amino-benzotriazole is also 5-amino-triazole."
Instead of this you should read:
1,2,4-triaminobenzene when diazotized in position 1 or 2 turns into 5-amino-benzotriazole what is the same as 6-amino-benzotriazole because of the
resonance of the triazole ring.
1)I forgot benzo before triazole
2)I forgot that after triazole formation, you of course have a one extra atom what shift the 4 amino in 5fth position.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Several members have asked me where the tenzotriazole cames from; well it is available once again on Ebay, in the UK at least, at a very reasonable
price in quantities from 200g to 2kg. At 17 GBP for 500g its pretty cheap.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by Boffis  | | Several members have asked me where the tenzotriazole cames from; well it is available once again on Ebay, in the UK at least, at a very reasonable
price in quantities from 200g to 2kg. At 17 GBP for 500g its pretty cheap. |
Photographers Formulary and ArtCraft both carry benzotriazole.
It is useful for preserving the silver coating on silvered telescope mirrors (amateur telescope mirror makers still do this sometimes).
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
As part of my ongoing work on benzotriazoles I just tried to prepare some 1,2,4-triaminobenzene by the reduction of 2-nitro-1,4-diaminobenzene
(commercially available as a hair dye ingredient). I used 5.01g of the nitro-compound, 8.02g of iron powder and 45ml of 30% HCl dilute with a further
45ml of water. The nitro compound was dissolved in the diluted HCl giving a brown solution and then the iron powder added in small amounts with gentle
warming for about an hour. The slurry was then filtered and poured into 400ml of 4% sodium hydroxide solution. The idea was that the ferrous hydroxide
and free amine could be filtered off together and the amine stripped out with ether or similar. Unfortunately the initial dark green flocculent
precipitate rapidly redissolved to form a dark green almost black inky solution.
My conclusion is that the resulting o-diamine forms an alkali stable complex with ferrous iron. Bit of a bugger! Interestingly in Org Synth they have
a reduction of 2-nitroaniline with zinc in alcohol and NaOH so I will try this on next.
[Edited on 2-11-2015 by Boffis]
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Boffis: "commercially available as a hair dye ingredient" - how should we interpret this?
Did you buy a box of hair dye from the supermarket and this compound was in one of the prepacked sacks/ plastic droppers/ whatnot? Which brand of hair
stuff did you use?
Was this compound prepacked in pure form or one should expect a bit more work, maybe an extraction of some sort?
Or you bought it from a chem. supply house and the above quote was just a side note?
Interesting what you do with these triazoles and unveiling a possible otc source would be a nice teaser too. :-)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Pumukli. back in 2011 /2012 numerous lots of this compound appeared on ebay. I bought 2 lots from different suppliers. One lot 100g was from a German
laboratory supplier but the other also 100g came in a plastic bag with a home-made label. Curiously the later material was much better quality because
it was in the form of relatively coarse crystals that oxidize less quickly.
I wonder what it might be used for and the only purpose that came up when I searched was "used for dye and chemical manufacture, specifically for
dying hair" and indeed most of the data I have on the compound comes from a product data sheet for a producer who produces chemical specifically for
this purpose. It now seems to have been phased out due to potential cancer concerns.
I am interested in the potential ligands derived fom triazoles and other heterocyclics. First though I need to find ways to produce the basic building
blocks which are 4 and 5 substituted 1,2,3 triazoles etc.
By the way did you know that there is a naturally occurring copper II amminotriazolato complex from a Chilean gauno deposit. I am not sure from the
formula whether it is a 1,2,3 or 1,2,4 triazole but it acts both as ligand and anion producing a univalent copper II complex cation. If you are
interested search for "chanabayaite". It occurs associated with the another copper heterocyclate mineral that contains cyanurate ions too.
|
|
|
| Pages:
1
2 |
|