learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
naphtol - alkali fusion - water?
This is from Gatterman
| Quote: |
MATERIALS required: 70 g. (0-3 mole) of sodium naphthalene-
3-sulphonate, 210 g. of sodium hydroxide, 20 c.c. of water.
The sodium hydroxide is powdered and, along with the water, is heated with stirring in a copper or nickel crucible to 280°.
As soon as the temperature has reached 280° the sodium
naphthalenesulphonate is added rather rapidly with stir-
ring ;
|
My question : what's the point of adding water? Wouldn't all the water be gone when the water/NaOH mix is heated? Surely at 280C all the water is
gone?
rest of the procedure
| Quote: |
heating is continued with a somewhat smaller flame and the temperature is maintained between 260° and 280°. After all the salt has been added, the
flame is raised somewhat, causing the molten material to become more viscous as steam is evolved and bubbles are formed. Finally, at 310°, the
reaction proper sets in. After the temperature has been kept at 310°-320° for about five minutes the material becomes more mobile and the reaction
is over.
|
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
Yes, I saw that, but I'm not sure how it answers my question?
As far as I can tell, the first step is to add 10% of water to the NaOH, and then heat the mix to 280C - Wouldn't the water boil off?
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
It looks like the water may act as a mixing agent, bringing the reagents in more intimate contact with each other. The mention of the viscosity of the
melt as the water leaves is a hint that melting first and mixing second doesn't work nearly as well.
|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
But as I understand the instructions, first NaOH and water are heated alone. Will the NaOH be molten at 280? (MP is 318...)
Anyway, I'm going to try it now.
(What I thought after making my 2nd post is that perhaps 'wet' NaOH won't get 'dry' at 280 and the MP of 'wet' NaOH is lower than 318?)
[Edited on 20-11-2012 by learningChem]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
You also might want to check out the procedure shown in Fierz-David, p. 188, in the forum library.
NaOH fusions are interesting, and challenging due to the high temperature required. I was only partially successful at making resorcinol from sodium
benzene m-disulfonate even though I had proof that my homemade disulfonate was quite pure. I made a small molten metal bath for this
purpose.
I would also like to make beta-naphthol via NaOH fusion. Please report your results.
[Edited on 21-11-2012 by Magpie]
[Edited on 21-11-2012 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
Magpie, thanks for the pointer! I had downloaded that book but forgot about it - I'll check it now.
Anyway, first attempt : mostly a failure.
I used 7g NaOH, ~1g H2O, 2.1g Na sulphonate.
I used a stainless steel cup as crucible. The amount of NaOH was too small and it barely covered the bottom of my crucible.
I put the thermometer in a copper tube closed at one end, but didn't realize that it wouldn't be easy to handle because the tube gets...hot. Also,
Gatterman says to put a bit of oil inside the tube, but the only oil I have at hand is sunflower oil. I'm not sure if it can take ~320C(I'll check
that tomorrow), so I skipped that part. Also the molten hydroxide barely covered the tip of the tube.
Long story short, I wasn't able to stir things easily or get accurate temperature readings.
The NaOH - 10% water mix is a slurry at room temperature. At ~220 - 250 it seems to melt somehow. The melt got yellow. I suppose something in the
crucible is reacting with it...damn.
Anyway, I added the sulphonate when the thermometer reached ~280. In something like 30 seconds (I think) there was a grey oil floating on top of the
NaOH. I let it cook for 3 or 4 minutes more but didn't see any frothing. The oil started to get darker so I removed the flame. I thought it was
getting charred.
I let the melt cool in the crucible and then dissolved it in 50ml of water (or more). I got a brown mess that seemed to contain some kind of
suspension. Neutralized it with HCl, the water moslty cleared and a dark brown precipitate appeared. I don't think it's naphthol though. Or maybe
there's a bit of naphthol but most of it is charred.
Anyway, I'm going to try again tomorrow with a proper thermometer setup (picture) and a bigger batch.

|
|
|
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
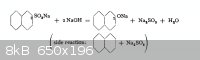
reaction
|
|
|