roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
imine capture
Flummoxed by the tenacity of the beta hydroxyl group of the Phenylpropanolamine series, and not having TV or other amusements I was wandering
around the vaults of erowid, where some would think i should have stayed.
But quickly consider the German patents
[1] German Patent 3,026,698
[2] German Patent 3,200,232
The authors are dehydrating ephedrine using 79% sulfuric acid with ZnCl2.
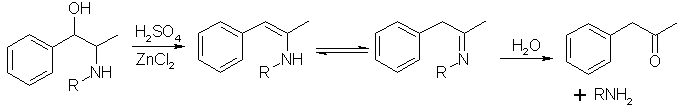
http://www.erowid.org/archive/rhodium/chemistry/phenylaceton...
The intermediate enamine/ imine is the target of theoretical interest.
Now temperature would play a crucial role i think. Certainly over heating will cause loss of the nitrogen .
On the cold extreme ...look..... another mechanism
(+)-Pseudo-ephedrin-O-sulfuric-acid-ester from (-)-Ephedrin. Hermann Emde, Helvetica Chimica Acta, p.402 (1929).
To 100 grams of ice-cold concentrated sulfuric acid situated in a beaker, 20 grams of natural (-)-ephedrine hydrochloride is added in about 40
portions within 10 minutes, whereupon hydrogen chloride gas is vigorously evolved. ........whereby beautiful white crystal needles of
(+)-Pseudoephedrine-O-sulfate ester precipitates. The crystals are filtered with suction in a small Buchner funnel, washed with a little cold water,
and after air drying the yield is approximately 10g of (+)-Pseudoephedrine-O-sulfate ester.
In the cold dehydration does not occur but an ester is formed.
So for now I will leave it at that. But I am trying to envision a theoretical cell of platnium which may cause the reduction of said imines with
possible passing of hydrogen instead of steam as in the german approach.
I must go check on my methanol extracts.... It extracts chlorophyll so beautifully.
|
|
|
killer_lapin
Harmless

Posts: 47
Registered: 23-7-2010
Location: Qc, CAN
Member Is Offline
Mood: No Mood
|
|
may be if the reaction is caried in different solvent like THF Glyme there would still be the first reaction but not the hydrolysation of the imine if
you add a water adsorbant in the reaction like MgSO4( shouldn't contaminate the reaction)
You can also make the alcool into a leaving groupe with a tosylate and use a base at high temperature to form the enamine without any water. And if
you use NaOH as a base to kick the tosylate the side reaction yould produce another isomer of ephedrine.("starting material")
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
A more interesting dehydration might be.......
C5H6-CH2-CH(NH2)-CH2OH----------> ?
Ephedrine is no longer easy to come by. Phenyl-Alanol can be readily produced by the reduction of Phenyl-Alanine or its Esters.
Does anyone have insight into this reaction sequence?
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
Well, thank you for the insights and parallels.
The only other follow up link i would like to post to close out the subject is
http://onlinelibrary.wiley.com/doi/10.1002/jctb.503300150/ab...
Catalysed hydrogenation of hydroxynitroanthraquinones in strong acid
Abstract
The catalytic hydrogenation of hydroxynitroanthraquinones such as 1,8-dihydroxy-4,5-dinitroanthraquinone over either palladium-carbon or
platinum-carbon catalysts proceeds readily in 75% sulphuric acid at 125°C during 6–12 h at 100 kPa pressure to give the corresponding
aminohydroxyanthraquinones in high yield.
$&$&$&$&$&$&$&$&$&$&$&$&$&$&$&$&$&$&$&$&$&$&$&$
There's plenty of other subject matter, but the above link highlights, again, a strong sulfuric acid system.
I dont have access to UV spec equipment at this time, but
one can easily in vision a interesting absorbance study at increasing temperatures. The imine/enamine should have an absorbance around 390nm, and
should become detectable.
At this temperature one could proceed with further study.
Do you think one of these UV flashlights could be of crude help?

|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Don't know for sure, but they sure illuminate nail fungus. My UV flashlight takes 3 AAA batteries, and at the moment, it is working well.
|
|
|