Nickdul
Hazard to Self
 
Posts: 96
Registered: 13-2-2012
Location: Bulgaria
Member Is Offline
Mood: No Mood
|
|
Synthesis of N-(4-Hydroxyphenyl)glycine (or related photography processing chemicals)
Well. allo me to introduce myself. I'm Nick,16, from a small village in Bulgaria. My interest in (very basic) chemistry led me to take up analog
photography some time ago. Basically, I* make my own AgX emulsions and change commercial products to suit my needs. Currently, I'm fiddling with
something called spectral sensitivity, which as you probably have guessed/know is how an emulsion responds to the various wavelengths in the spectrum.
A simple, blue sensitive emulsion is principally sensitive only to light in the 350-470nm region - or UV to violet-blue. Now, by adding various dyes,
I can impart various other sensitivities. The way those dyes functions act is complex, but basically their molecules adhere to the AgX crystals and
have the ability to "donate"(I guess) an electron to the AgX crystal when stricken by light of a wavelength the silver halogens aren't naturally
sensitive to. These dyes are pretty special, and expensive(running anywhere from $50 to $1500 per gram).
However, perusing some old books has yielded valuable information as to alternatives to current dyes. erythrosine, eosin Y, and chlorophyll are
weaker, but still viable spectral sensitizers(SS). However, the books recommend the use of something called quinoline red(chinoline red/rot) and
cyanine blue.
Google searches only resulted in CAS numbers and an empirical formula of quinoline red - C26H19N2Cl, or a name -
1-[(2-chlorophenyl)(naphthalen-2-yl)phenylmethyl]-1H-imidazole. I got nothing on cyanin blue (also refferd to as chinoline blue), though.
So, anyone that has any idea how I could possibly synthesize/acquire these compounds, I'd be deeply grateful 
Nick
PP: Whilst researching the dyes I did come across a recipe for "azaleine"(??) procured by "reacting aniline with mercury nitrate or other nitrates"
although all formulas speak of the mercury nitrate.
Edit by Nicodem: Merged Nickdul's posts with the the other N-(4-hydroxyphenyl)glycine thread.
[Edited on 5/5/2013 by Nicodem]
|
|
|
mghis
Harmless

Posts: 2
Registered: 5-1-2013
Member Is Offline
Mood: No Mood
|
|
N-(4-hydroxyphenyl)glycine Preparation
Hello,
I am wondering how to synthesize N-(4-hydroxyphenyl)glycine, also known as photoglycine, used a few decades ago as a film developer. Below there is
the structure of this compound.
I looked it up on "The Merck Index", and it says is is produced with chloroacetic acid and 4-aminophenol with the following reaction:
Cl-CH2-COOH + HO-C6H4-NH2 ➞
HO-C6H4-NH-CH2-COOH + HCl
I also thought I could use an aryl halide, using the Ullmann reaction as described here: http://www.organic-chemistry.org/abstracts/lit3/169.shtm. I only have read the abstract, as the article is not free, but it seems neat and easy,
and has excellent yields. In this way I would use 4-bromophenol and glycine, catalyzing the subsitution with metallic copper, if I have understood
correctly.
Do you think that the synthesis I thought will work? In your opinion, what are the conditions of the first reaction? Are there other convenient ways
to prepare my compound?
Thanks for any answer or suggestion, and sorry for my poor English.
-- mghis
[Edited on 17-2-2013 by mghis]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mghis  | I also thought I could use an aryl halide, using the Ullmann reaction as described here: http://www.organic-chemistry.org/abstracts/lit3/169.shtm. I only have read the abstract, as the article is not free, but it seems neat and easy,
and has excellent yields. In this way I would use 4-bromophenol and glycine, catalyzing the subsitution with metallic copper, if I have understood
correctly.
Do you think that the synthesis I thought will work? |
Maybe, but a reaction of glycine with p-bromophenol or p-iodophenol would likely be unselective because phenols are also
nucleophiles able to participate in the condensation. Otherwise, glycine is an excellent ligand for promoting the Ullmann condensation and the
reaction could potentially work well with a large enough excess of glycine due to absence of any additional base that could mess up things by phenolic
group deprotonation. You would also need to use a minimum amount of a solvent, because the phase behavior properties and solubility of glycine are
very different to the amines used in that article. Water might actually work because p-aminophenol is soluble in it at those temperature. Otherwise, I
would suggest NMP, DMF, DMAc, DMI or DMPU.
Also, UTFSE on the topic of the Ullmann condensation - there were posted references to review articles and there is plenty of discussion about it on
the forum.
| Quote: | | In your opinion, what are the conditions of the first reaction? |
I don't know. You tell us, you have the reference.
| Quote: | | Are there other convenient ways to prepare my compound? |
I doubt there is anything more convenient than the alkylation of p-aminophenol with chloroacetic acid. Both of these reagents are cheap. But feel free
to search the literature for alternatives.
Welcome to the forum.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
bananaman
Harmless

Posts: 19
Registered: 29-1-2013
Member Is Offline
Mood: No Mood
|
|
I tend to believe that there is a need for some protection chemistry to be performed on glycine and 4-bromophenol before starting this experiment.
Additionally, Buchwald-Hartwig amination conditions might be better for performing this coupling work. 
|
|
|
mghis
Harmless

Posts: 2
Registered: 5-1-2013
Member Is Offline
Mood: No Mood
|
|
Thank you both for answering.  I will indeed search the forum for the Ullmann
reaction, as suggested, and look up the Buchwald-Hartwig amination, that I didn't know. I will indeed search the forum for the Ullmann
reaction, as suggested, and look up the Buchwald-Hartwig amination, that I didn't know.
|
|
|
Nickdul
Hazard to Self
 
Posts: 96
Registered: 13-2-2012
Location: Bulgaria
Member Is Offline
Mood: No Mood
|
|
Synthesis of N-(4-Hydroxyphenyl)glycine
Hi,
I'm trying to produce hydroxyphenyl-glycine for my photographic work, as it is a favored developing agent in certain situations.
I know the preferred method is reacting p-aminophenol with chloroacetic acid to yield photoglycine and HCl. However, the Merck index goes so far, and
the only reference it lists that I could access is a German article (1884) that I can barely interpret. I ran some searches on this forum and some
photographic ones, and found little useful information.
Now, I have a solution that is purportedly 80% MCA, but it might as well be 80% TCA, owing to a vague name on the label. What I tried was a small
scale experiment with the little info I have. I mixed 300mg 4-aminophenol, 400mg sodium acetate( as per the German article) and 260mg equivalent of my
M/TCA solution. , water was added to approx. 2,5ml. The solution was gently heated, and at around 45-50C, the p-aminophenol dissolved, leaving behind
a pale-yellow solution. Upon cooling to 5C, needle-like clear crystals precipitated and were filtered off. I reacted the filtrate with NaHCO3 to
neutralize the acids, expecting the neutral pH to cause more glycin to precipitate (literature suggested it was more soluble in acidic soln).
The crystals seemed to dissolve easily in water, contrary to the sparingly soluble glycin. So, I took some of the solution and tested it for
p-aminophenol by adding some plain bleach(hypochlorite) and observed the characteristic purplish color that i had observed with some pap-HCl.
Now, I have no firm foundation in organic chemistry, beyond simple hydrocarbons, ketones, ethers, etc, so any help would be welcome 
What I hypothesize, possibly incorrectly, is that the old-ish TCA/MCA soln that I have may have decomposed somewhat( it had a faint acetic
acid/acetate odour) and the p-aminophenol I added was simply converted into the HCl salt.
Here are the structural formulas of the compound that I have, and the one I want to synthesize:

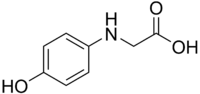
So, if you have any ideas, I'd be extremely happy to hear them and try them out  Also, if anyone has access to the following article, I would leap with joy
Also, if anyone has access to the following article, I would leap with joy 
Thanks in advance,
Nick
|
|
|
Nicodem
|
Threads Merged
5-5-2013 at 11:48 |
Nicodem
|
Threads Merged
5-5-2013 at 11:50 |
|