| Pages:
1
2
3 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
A very nice and to the point experiment, indeed. This also appears one of the better ways to prepare ClO2.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I also tried the experiment with KClO3 and H2SO4. I knew this one already, but I tried it again to watch the precise color of the liquid obtained in
this experiment. This liquid, however, is really different from the brown color I obtain in the other experiments. KClO3+H2SO4 gives a bright orange
liquid and not a dark brown liquid. It gives ClO2 (visible yellow gas is produced and floats around the pile of KClO3 and the drop of liquid) and it
gives white fumes. I think it is disproportionation of HClO3 to HClO4 and ClO2 and the orange color of is due to concentrated ClO2, dissolved in the
acid.
@eddygp: I do not understand your remark. The equation, provided by blogfast25, is perfectly balanced.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Eddygp  |
Uhh that reaction cannot be balanced. I have tried to balance similar equations with HClO2 yielding HCl and ClO2 but I can't balance any...
|
Like all redox reactions it helps a lot if you break them down into oxidation and reduction ‘half reacions’. Here:
(1) HClO2 === > ClO2 + H+ + e- (oxidation to Cl (IV) oxide)
(2) HClO2 + 3 H+ + 4 e- === > Cl- + 2 H2O (reduction to chloride)
4 x (1) + 1 x (2) and mild rearranging then yields the balanced result.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Quote: Originally posted by woelen  |
@eddygp: I do not understand your remark. The equation, provided by blogfast25, is perfectly balanced. |
My bad, I don't see why I said that... I thought there was a hydrogen missing or something. Sorry.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I also tried what happens when a big drop of Br2 is added to a solution of 30% NaClO2. When this is done, then the liquid becomes very dark, nearly
black and an intensely colored yellow gas mix slowly appears above the liquid. The bromine quickly dissolves in the solution of NaClO2 and the entire
liquid becomes very dark. The dark liquid slowly produces fairly big bubbles of gas.
I lighted the open end of the test tube with the dense yellow gas mix and this gives an impressive sound, the well-known WHOOSH sound when
hydrogen/air mixes are ignited. The sound, however, was powerful (quite scary, I held the test tube in my hand, but wrapped in a 2 cm thick layer of
towel). After the 'burning' of the ClO2, a pale brown/yellow color remained in the gas-mix in the test tube (due to some left over bromine vapor) and
slowly the gas-mix above the dark liquid turns yellow again.
Most scary was that after the lighting of the ClO2 in the test tube, there was a strong crackling noise just above the dark liquid. The dark liquid
was bubbling and each time when a bubble made it to the surface, it exploded with a loud CRACK noise. Probably this was due to the high temperature in
the gas mix. I was afraid that the entire dark liquid would explode, but that fortunately did not happen.
So, Br2 also is capable of oxidizing ClO2(-) and then even more of the intensely dark colored compound is formed.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Woelen:
I not sure I that adding Cl2 to aqueous solution eliminates the acid question as some Cl2 can react as follows:
Cl2 + H2O <---> HCl + HOCl
-----------------------------------------------
I would also add, in my opinion, that the picture you provided appears to support my hypothesis as any Cl2 formed in the test tube (per decomposition
or disproportionation reaction cited previously) would bubble to the top. Then, again per the reaction cited above, a locally higher concentration of
HCl could form removing the dark brown Cl2O3 species per the reactions:
Cl2O3 + H2O = HOCl + HClO3
HOCl + HCl = Cl2 + H2O
---------------------------------
Net:
Cl2O3 + HCl = Cl2 + HClO3
Conversely, the lower half of the test tube is where the more intense darker color is expected (lower local HCl concentration), and it appears also to
be so observed.
--------------------------------------------------------
[EDIT] Here is an interesting tests consistent with my reaction chain model and properties of compounds formed:
First, the simpliest test, with time/light HOCl decomposition should increase forming HCl, and the solution's color could regionally be impacted.
Similarly, adding a drop of H2O2 should more rapidly form O2 and remove color.
Second test, cooling and warming a fresh solution should affect color.
[Edited on 19-5-2013 by AJKOER]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  |
So, Br2 also is capable of oxidizing ClO2(-) and then even more of the intensely dark colored compound is formed. |
Very interesting but can we know for sure it is the same compound generated with Br2 as with Cl2? As such, in the absence of identifying evidence like
UV/VIS data, I say 'no'. It would se SOOO interesting to run these reactions at low concentration in cuvettes and take spectra, possibly as a time
series?
[Edited on 19-5-2013 by blogfast25]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
With respect to the Bromine experiment, here is a reference ("The Reaction of Chlorine(III) with Bromine and Hypobromous Acid: Kinetics and
Mechanism", http://www.google.com/url?sa=t&rct=j&q=hclo2%20%2B%2... )
Some reactions presented include:
Br2 + ClO2− = Br2− + ClO2
Br2− + ClO2− = ClO2 + 2Br−
Br2 + Br− = Br3−
ClO2− + H+ = HClO2
H2O + Br2 = HOBr + Br− + H+
Br + Br− = Br2−
Br + Br = Br2
Br + ClO2− = Br− + ClO2
HOBr + HClO2 = BrClO2 + H2O
BrClO2 + ClO2− = 2ClO2 + Br−
where Br is a bromine radical and Br− is the bromide ion. I would add that bromine does not measurable dissolve in water until some bromide is
created, then forming the tribromide. Note, several of the reactions cite the formation of ClO2 as was observed.
Another source (http://pubs.acs.org/doi/abs/10.1021/es302730h ), which includes the pesence of Chlorine states:
"HOBr, formed via oxidation of bromide by free available chlorine (FAC), is frequently assumed to be the sole species responsible for generating
brominated disinfection byproducts (DBPs). Our studies reveal that BrCl, Br2, BrOCl, and Br2O can also serve as brominating agents"
As such, Br2O (and even perhaps, speculatively BrO2, formed by the action of Cl2O3/HClO2 on Br2O or BrClO2) could be responsible for the exploding
bubbles. The described pale brown/yellow gas was most likely BrCl described by Wikipedia as being brownish yellow.
[Edited on 19-5-2013 by AJKOER]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I did another experiment, now using S2O8(2-) as oxidizer. This oxidizer has a very clean reaction:
S2O8(2-) + 2e --> 2SO4(2-)
No acids involved, no free halogens involved.
I expect that with chlorite the reaction is as follows: S2O8(2-) + 2ClO2(-) --> 2So4(2-) + 2ClO2
Now the result of my experiment:
- Prepare a 30% by weight solution of NaClO2
- Add some solid Na2S2O8 to this solution.
- As soon as the white solid is added, the solid becomes covered by a yellow/ochre layer, which soon becomes dark brown. Initially, the layer has a
color like mustard and not the bright orange which appears in the KClO3/H2SO4 experiment as suggested by garage chemist. By slowly swirling the
solution, nice brown 'schlieren' of dark solution are obtained. When swirled a little stronger, a homegeneous very dark solution is obtained.
- Wait some time: The reaction proceeds and goes faster. The liquid becomes nearly black and bubbles are produced. Above the liquid there is a strong
and deep yellow color of (nearly) pure ClO2.
At this point I added a lot of cold water. I had the unpleasant feeling that the reaction went too fast and I did not feel comfortable with it. The
concentration of ClO2 was very high and I feared a sudden explosion, hence the quenching in a lot of water.
This experiment nicely shows that just ClO2 without acid and without free halogens produces the dark brown color in a solution of NaClO2. This
experiment also tells more than the experiment with Br2, because of the clean reaction of peroxodisulfate in redox reactions.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Here is a new theory based on the chemistry presented in the patent (see "Method and composition for in-situ generation of chlorous acid" by Roy W.
Martin, link: http://www.faqs.org/patents/app/20120107418 ) namely we are possibly viewing an aqueous intermediary compound, to quote from the patent:
"[0049] Where X represents Bromine (Br) and Chlorine (Cl)
2ClO2- + X2(g) → 2ClO2 + 2X- (1a)
2ClO2- + HOX → 2ClO2 + X- + OH- (1b)
[0050] However, these equations give a simplistic representation of the generation process. Considering the mechanism of these reactions is important
for a better understanding of the details of the generation process and the invention. The intermediate species (XClO2) forms in these reactions. This
intermediate may react to give ClO2 or chlorate ion according to Equations 3-4.
Where X represents Bromine (Br) and Chlorine (Cl)
X2 + ClO2- → [XClO2] + X- (2)
2[XClO2]→2ClO2 + X2 (3a)
[XClO2] + ClO2- → 2ClO2 + X- (3b)
[XClO2] + H2O → ClO3- + X-+ 2H+ (4)
[0051] Equations 3a-b are important at high concentrations when the formation of XClO2 is rapid. On the other hand, Equation 4 is more important when
the formation of XClO2 is slow, such as at low reactant concentrations or high pH values. "
That is, my new suggested theory is that one is viewing the intermediary compound Dichlorine dioxide, Cl2O2 in solution (or BrClO2) created by the
action of Chlorine (or Bromine), per the reactions cited above, per the high concentration reactions with Chlorine (or Bromine), namely for NaClO2, as
an example:
3 Cl2 + 3 NaClO2→ 3 [Cl2O2] + 3 NaCl (per 2)
2[Cl2O2] → 2ClO2 + Cl2 (per 3a)
[Cl2O2] + NaClO2 → 2ClO2+ NaCl (per 3b)
or net:
4 NaClO2+ 2 Cl2 → 4 ClO2 + 4 NaCl
or, rescaling:
2 NaClO2 + Cl2 → 2 ClO2 + 2 NaCl
in agreement with reaction (1a).
Note, per Wikipedia (http://en.wikipedia.org/wiki/Cl2O2 ) Dichlorine dioxide is a naturally occurring reaction intermediate formed through the combination of two
chlorine monoxide radicals during atmospheric photochemical reactions.
The cited patent above describes sample solutions with varying colors of gold, darker gold and amber.
[EDIT] This model suggests that on heating the solution's color should vanish (along with the intermediary), and with varying solutions at different
increasing temperatures, the color should fade more rapidly per the relative temperature.
Also, with respect to the reaction: S2O8(2-) + 2ClO2(-) --> 2So4(2-) + 2ClO2, the short answer is that this could be a net reaction also.
[Edited on 19-5-2013 by AJKOER]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
I'm afraid to propose a stupid thing,but what is the color of solution if you pass chlorine gas into HCL?
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
papaya I have done this as part of an unrelated experiment and the solution turned slightly yellow if anything.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
That I asked to know what is the color of HCL3 because I thought in high concentrations it may become darker.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by papaya  | | That I asked to know what is the color of HCL3 because I thought in high concentrations it may become darker. |
"HCL3"? What's that supposed to be?
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
That's a chlorine analog of H[J3] , there are polyhalide anions like in KJ*J2 whichmore precisely is K[J3] complex...
https://en.wikipedia.org/wiki/Polyhalide
[Edited on 20-5-2013 by papaya]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AJKOER  |
That is, my new suggested theory is that one is viewing the intermediary compound Dichlorine dioxide, Cl2O2 in solution (or BrClO2) created by the
action of Chlorine (or Bromine), per the reactions cited above, per the high concentration reactions with Chlorine (or Bromine), namely for NaClO2, as
an example:
[Edited on 19-5-2013 by AJKOER] |
The author is clearly freewheeling with regards to 'XClO2' and presents not a shred of evidence for its existence, instead piling up hypothesis upon
hypothesis. Not a spectrum in sight, let alone an elemental analysis of 'XClO2', stoichiometrical proof or a MW determination. It's all conjecture.
NOT impossible but UNPROVED.
Patents, by their very nature, can be very deceitful.
[Edited on 20-5-2013 by blogfast25]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Blogfast:
Thanks for asking for further research on Cl2O2. I found the precise reactions (and more) discussed in detail in a good book "Inorganic Chemistry",
edited by Arnold F. Holleman, Egon Wiber, Nils Wiberg at link: http://books.google.com/books?id=Mtth5g59dEIC&pg=PA459&a... at the bottom of page 460.
The author clearly states that Cl2O2 (or ClClO2) is actually reputedly formed in several reactions of concern here. It also is stated to readily
decomposes. There is, however, a slow rate determining step that may keeps the dichlorine dioxide visible for awhile depending on reaction conditions.
For example:
HClO2 + HClO2 --H+--> HClO + HClO3 (slow rate determing step)
HClO + HClO2 --> ClClO2 + H2O
ClClO2 ---> 1/2 Cl2 + ClO2 (rapid)
Net to this point:
3 HClO2 --H+--> HClO3 + H2O + 1/2 Cl2 + ClO2
There is also a reaction forming more ClClO2 from the Cl2:
1/2 Cl2 + 1/2 HClO2 --> HCl + ClClO2
The author also directly states that the reaction cited in the patent (Equation 10 on page 460):
2 NaClO2 + Cl2 --> 2 NaCl + 2 ClO2
has ClClO2 also as an intermediary.
------------------------------------------------------
OK, so the chemistry look good, but as to whether it is the actual cause of the color, I am not certain, but it appears to qualify as a possible
candidate.
[Edited on 20-5-2013 by AJKOER]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Mind you, ClClO2 is not the same compound as the symmetrical Cl2O2 in the wikipedia link that you posted earlier. It looks like it would involve one
chlorine atom with nominal oxidation state +1 and another at +3, which at least looks interesting as a possible charge transfer complex.
Another relevant paper I found, with a nice set of cited work that I haven't time to look through right now:
http://hopf.chem.brandeis.edu/pubs/pub293%20rep.pdf
The less you bet, the more you lose when you win.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Bbartlog:
I have looked at the excellent research paper you supplied.
I also found interesting that Cl2O3 can be created via the pathway:
ClO2- + Cl2O2 --> Cl- + 2 *ClO2
HClO2 --> *ClO + *OH
Leading to:
*ClO2 + *ClO --> Cl2O3
so I would add Cl2O3, as one of the "longer-lived" (per the authors words on the first page) intermediary species back on the candidate list as well.
Also, around equation E3 on page 6970, the authors cite three reason for re-introducing Cl2O3.
Bottom line, for this well-studied decomposition reaction the species observed are HOCl, Cl2O2, Cl2O3, *ClO and *OH. And, as the radical *ClO interact
with itself to form the symmetric form of Cl2O2 in a termination step, I think we have at least mentioned all the possible candidates responsible for
the color.
-------------------------------------------------
Here is the last candidate and may even be the answer. The conditions are some ClO2 is formed in solution containing a high level of chlorite. Some
reactions:
HClO2 <--> H+ + ClO2-
ClO2 + H2O <--> HClO2 + HClO3
[EDIT] My research (http://www.google.com/url?sa=t&rct=j&q=hydrolysis%20... ) notes an interesting property of ClO2, to quote:
"One of the most important physical properties of chlorine dioxide is its high solubility in water,
particularly in chilled water. In contrast to the hydrolysis of chlorine gas in water, chlorine dioxide
in water does not hydrolyze to any appreciable extent but remains in solution as a dissolved gas
(Aieta and Berg, 1986). It is approximately 10 times more soluble than chlorine (above 11°C), while
it is extremely volatile and can be easily removed from dilute aqueous solutions with minimal
aeration or recarbonation with carbon dioxide (e.g. softening plants). Above 11 to 12°C, the free
radical is found in gaseous form."
As such, the last hydrolysis reaction above is pretty much to the left and with a high chlorite concentration, basically just unreactive ClO2 gas in
water.
This site notes that at high concentrations of ClO2, a significant brown appearance (link: http://www.linkedin.com/groups/Can-anyone-explain-difference... ). To quote:
"H. Peter H. • You are walking a dangerous line when you see brown fumes - chlorine dioxide is not just toxic, it is also explosive. In dilute
solution or as a dilute gas, it is yellow, and with increasing concentration, it is getting more and more orange-coloured. When you see brown, it is
reason to be alarmed."
So the answer could just be free ClO2. A test for this would be to check the color sensitivity to HCl, HOCl and/or Cl2 as these would promote the ClO2
hydrolysis by attacking the products. Another test, add a few drops of seltzer water (CO2/H2O). If the color rapidly vanishes, it is ClO2 and not an
intermediary.
[Edited on 21-5-2013 by AJKOER]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
AJ: I didn't really ask you for anything but anyway.
Although all this makes 'Cl2O2' (but which of the three?) a potential candidate to explain the dark substance, without more direct 'fingerprinting'
evidence re. the exact nature of that substance everything here remains speculation. Unless woelen can come up with indirectly corroborating
experiments that an 'XClO2' type species is at play here, we've basically come to the end of the line as fat as I'm concerned.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Reaction ClO2 + chlorite -> "deeply brown something" is known (in chemical literature).
But I do not want to break up the party here.
Have a nice searching (and finding).

Слава Україні !
Героям слава !
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Indeed, the combination of ClO2(-) and ClO2 causes the brown color. If you prepare a neutral solution of ClO2 in water then the solution becomes deep
yellow. If you want to repeat this experiment, be careful! let concentrated ClO2 diffuse into a small pool of water, do not suck ClO2 in syringes
or other plastic/rubber things!
If, however, NaClO2 is present in the water, then the color is different, it is more brown/mustard. I did not do experiments with very concentrated
solutions of ClO2 with NaClO2, because that requires handling larger amounts of high concentrations of ClO2 and I fear its explosive power too much.
The experiments I did with lower concentrations, however, are quite convincing and the other experiments I did before also give very good evidence
(especially the one with Na2S2O8 which affords production of ClO2 only without adding any acid in the mix).
I also read about this subject on internet. Remarkably, the brown compound is mentioned nowhere in scientific documents, which I can access. I'm quite
sure other people must have noticed it as well, but I did not find any scientific paper describing it. I found it mentioned once, not in a reputable
paper, but on some site which describes making MMS (a miracle mineral solution which is supposed to be good for almost everything and more of that
kind of crap). This site warns for the brown solutions and the yellow gas, telling that it can explode and that it is dangerous and if this color is
observed that the concentration used in the preparation is way too high. More serious documents, however, describe single electron transfer reactions
in redox systems:
ClO2(-) <---> ClO2 + e(-)
This reaction occurs very easily, according to literature and can go in both directions. Many oxidizers easily make ClO2 from ClO2(-) and many
reductors easily make ClO2(-) from ClO2.
I have the impression that the brown color is due to some single electron transfer reaction between ClO2 and ClO2(-). I can imagine that ClO2 and
ClO2(-) become bonded to each other in some way and that the electron 'flips' from one side to the other at extremely high frequency (i.e. a resonance
structure is formed which at least partially overlaps both ClO2-entities) and that this process leads to strong absorption in the visible spectrum
(due to the relatively long distance, covered by this resonance system). I, however, did not find a true reference which confirms my theory, nor do I
have the equipment to validate this. My measurement devices are my eyes and other more advanced equipment I have not at home for doing this kind of
observations.
I speculate that this species will have a structure like O2Cl...ClO2 with ... being the special bond which is formed by the lone electron of ClO2 on
its Cl-atom and a free electron pair of ClO2(-), also on its Cl-atom. The molecular orbital may extend even further, into the oxygen parts of the
molecules.
As I said, there is quite some speculation from my side, but given the limited resources I have, I think it is the best I can offer.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
I think there may also be an interesting safety question here as well. Obviously, dissolving a large amount of ClO2 in water can form a
dangerous/potentially explosive solution. However, in the presence of a high level of chlorite, much less ClO2 may be able to produce a similar
colored solution (or, as I would describe it, per a visible small equilibrium amount of free ClO2 effected by a limited hydrolysis in the presence of
a soluble chlorite). The safety question is does this latter solution present the same level of danger, or is this the case of a false positive test?
My suspicion per Patent US 4892148 A at http://www.google.com/patents/US4892148 from the example cited below to quote:
"EXAMPLE 3
A granulated Sodium Chlorite was dissolved in water to form a 48% sodium cholite solution according to standard published data on solubility of Sodium
Chlorite. This solution was then combined with a solution of 88% lactic acid. An immediate reaction occurred forming a deep brown solution. This
solution was tested and the presence of ClO.sub.2 was detected. No attempt was made to ascertain the ClO.sub.2 ppm of this solution."
is that the solution is not dangerous (in other word, a false positive) given the particular nature of the patent involving bulk mixing for oil
recovery operations.
[EDIT] Further, given that the solution above has high concentrations of chlorite (from aqueous NaClO2) and HClO2 (via the lactic acid and NaClO2
reaction), it is also my opinion that aqueous Cl2O3 (the acid anhydride of HClO2 isolated as a dark brown explosive solid) is as plausible an
explanation for the immediately observed deep brown color as is ClO2, being a yellowish-green gas which crystallizes as bright orange crystals at
−59 °C, which "in aqueous solution, depending on concentration, the colour varies fiom green-yellow to orange-red" per http://www.google.com/url?sa=t&rct=j&q=%22chlorous%2... page 11. I would also note that I would suspect ClO2 to be present in much smaller
amounts via decomposition/disproportionation reactions, in this particular example.
[Edited on 23-5-2013 by AJKOER]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
You are never going to get an explosion from a solution of sub 10% of anything.
Water is a fantastic thermal buffer.
Mixing 10ml of 35% hydrogen peroxide with 30ml of 10% sodium hypochlorite, roughly stochiometric proportions, produces a vigorous effervescence of
oxygen and a very hot solution but it does not explode.
A mixture of 35% hydrogen peroxide with 98% sulphuric acid, acid Piranha, can explode in contact with acetone, etc but it not an aqueous solution.
The fact is that; solid or solution, and if a solution, concentration play an important role in a materials properties.
|
|
|
phlogiston
International Hazard
    
Posts: 1376
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Quote: Originally posted by woelen  | | I can imagine that ClO2 and ClO2(-) become bonded to each other in some way and that the electron 'flips' from one side to the other at extremely high
frequency (i.e. a resonance structure is formed which at least partially overlaps both ClO2-entities) |
It is incorrect to think of molecules rapidly switching between resonance structures. This concept originates from quantum chemical descriptions of
molecules. The molecule is instead a static object (in the sense that no bonds are broken or formed, i.e. all electrons remain in their respective
orbitals). Intuitively, you can view a molecule as being a weighted average of all the resonance structures that you can draw. The structures with the
lowest energy are more likely and contribute more, (and very unlikely structures contribute very little).
As an example, consider the nitrate ion:
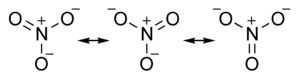
In reality, the negative charge on the oxygen atoms is static in time and distributed equally among the oxygen atoms. The N-O bonds are not a single
bond or a double bond but more like a 1.333 bond (in terms of electron density).
I am not qualified to comment on the likelyhood of your Cl2O4- structure, but interesting observations, as always.
[Edited on 23-5-2013 by phlogiston]
[Edited on 23-5-2013 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
| Pages:
1
2
3 |