| Pages:
1
2
3 |
RobertRobinson75
Harmless

Posts: 10
Registered: 8-5-2013
Member Is Offline
Mood: No Mood
|
|
I agree with this completely. As someone who is trying to teach themself and get into doing chemistry at home, it's very off-putting. I can't get a
condenser or still head where I live as well, which is very frustrating.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
sciencesupply has an absolutely terrific chart for glassware for all states. here's the link:
http://sciencesupply.com.au/shop/content/2-legal-notice
|
|
|
skeletal-clown
Harmless

Posts: 30
Registered: 29-5-2013
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Thanks, that chart puts it all in a nicely set out form. I'd just like to mention however, that at least for Victoria the items in red are classified
as "category 3 precursor apparatus". This DOESN'T mean they are illegal, it just means that you have to either have an account with the company you
buy them from, or you have to sign an end user declaration. ( Drugs, Poisons and Controlled Substances Act 1981, page 180 and Drugs, Poisons and
Controlled Substances (Precursor Supply) Regulations 2010, page 9).
So long story short, in Victoria at least you can still own these items, you just have to jump through a few hoops.
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
My problem was I had the full established lab prior to 2004 (when the WA government enacted legislation making the possession of glassware illegal)
Even if I was prepared to give up amateur chemistry (not for nobody) what does the WA gov expect.That I'll throw thousands of dollars of glass and
gear in the garbage?
On the bright side for everybody else but WA and QLD it seems you can import all the equipment you want.No need for registered accounts or
EUDs.Nobody's business and no laws broken.
Sorry,I've taken us a long way off topic,but these things need to be bitched about.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
What if you consider making the needed ware yourself? I can do most processes fine with my stainless steel self made ware. Labware isnt illegal in
here, but there are no suppliers because nobody buys them.
|
|
|
skeletal-clown
Harmless

Posts: 30
Registered: 29-5-2013
Location: Australia
Member Is Offline
Mood: No Mood
|
|
@starman, I couldn't agree more, bitching is good!
@testimento, That is well beyond the reach of many amateurs (myself included), but could be a great way to obtain glassware in such places. I think
there's even a book on it in the SM library...
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Hi, could HCl be used instead of H2SO4? I've read on wiki that you need strong acid and that H2SO4 is usually used, but it doesn't say what other
acids can be used. Thanks in advance!
BR, xfusion
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I don't think so. The big advantage of H2SO4 is that it is a non-volatile acid. HCl is a very volatile acid. Quoting from Len1's procedure in
Prepublication:
"The main requirement is that the combined initial reagent water should not make the initial H2SO4 concentration less than about 70%, at which point
the boiling point of the mixed acid approaches the reaction temperature used in step 2 - and it will start distilling over with the products."
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
HCl is volative, as well as chloroethane (b.p.12.3°C vs 33°C for diethyl ether).
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
The volatility really has nothing to do with it.
Sulfuric acid is a dehydrating agent.
Obviously Hydrochloric acid contains a LARGE amount of water.
Quite the opposite of what you want.
In theory other acidic dehydrating agents like phosphorous pentoxide could be used. But sulfuric acid is actually easier to handle than that one.
Alumina can also be used as dehydrating agent at high temperatures. A better choice for this particular reaction is anhydrous sodium bisulfate. It
has less by products and is more readily available.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by macckone  |
A better choice for this particular reaction is anhydrous sodium bisulfate. It has less by products and is more readily available.
|
Are you speaking from experience or do you have a reference for that claim? Please provide further details.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
So, is the H2SO4 used because of its acidity or high dehydrating ability or both?
So, I could use solid acids, with dehydrating ability?
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
macckone, thionyl chloride dehydrates and contains acid, but EtOH + SOCl2 does nothing except of EtCl, and on dehydration you get ethylene, which
actually is an unwanted side-product even in the EtOH-H2SO4 method.
Nice idea about phosphoric acid, hovewer I don't know about its selectivity towards etherification and dehydration.
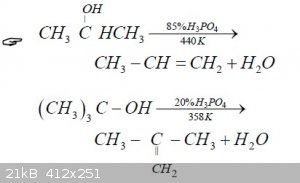
I know that ethyl sulfuric acid (EtSO4H) is an intermediate in the ether synthesis, but I have no idea about the mechanism (this one is probably wrong
https://commons.wikimedia.org/wiki/File:Acidic-ether-synthes... )
macckone, have you or somebody else ever succeeded in making ether using sodium bisulfate? I hear about it first time.
xfusion44, all known routes require some dehydration power (until you use already dehydrated precursors). As you might know, alumina (Al2O3) is a
close equivalent of sulfuric acid, but in the gas phase heterogenous reaction; they both are acidic and convert alcohols to olefin or ether, depending
on conditions.
[Edited on 29-5-2015 by byko3y]
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
@byko3y
So, it should work with alumina?
Thanks!
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
xfusion44, Diethyl ether synthesis on alumina is a well studied method. There are reports in the web on extensive researches of the process for
different catalysts and conditions, also, Ullman briefly describes the commonly used industial process (with usual alumina).
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
https://web.anl.gov/PCS/acsfuel/preprint%20archive/Files/49_...
Here's the paper in case you failed to find one. I want to emphasize that you can't obtain more than 60% ether per pass and the maximum conversion is
70%, but high conversion leads to higher amount o by-products, so the highest seelctivity on aluminophosphate catalyst requires implies conersion as
low as 50% per pass.
Sulfuric acid method gives perfect yield and selectivity when done properly.
An interesting result of the research: you actually need a lewis acid to synthesize the ether. Ethylsulfuric acid plays its role in case of sulfuric
acid method. I think sodium ethyl sulfate will work too, but it needs to be pre-formed, otherwise this stuff will hydrolize right away.
I did not find any paper that could describe ether synthesis via phosphoric acid even remotely. Phosphate esters are relatively stable and phosphoric
acid is a weak acid. Phosphoric acid on silica can lead to diethyl ether, but I think that on higher conversion rates it will just dehydrate it to
ethylene (phosphate is a bad leaving group).
Ionic resin is capable of promoting the etherification with excellent selectivity, but it either requires some drastic conditions http://qap2.onlinelibrary.wiley.com/doi/10.1002/aic.14497/ab... or is not usable because of polimerisation at higher temperatures.
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Quote: Originally posted by DJF90  | Quote: Originally posted by macckone  |
A better choice for this particular reaction is anhydrous sodium bisulfate. It has less by products and is more readily available.
|
Are you speaking from experience or do you have a reference for that claim? Please provide further details. |
Mellor inorganic chemistry is where I first read about it and experience. It produces less sludge. And it is available at most wally world stores.
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Quote: Originally posted by byko3y  | https://web.anl.gov/PCS/acsfuel/preprint%20archive/Files/49_...
Here's the paper in case you failed to find one. I want to emphasize that you can't obtain more than 60% ether per pass and the maximum conversion is
70%, but high conversion leads to higher amount o by-products, so the highest seelctivity on aluminophosphate catalyst requires implies conersion as
low as 50% per pass.
Sulfuric acid method gives perfect yield and selectivity when done properly.
An interesting result of the research: you actually need a lewis acid to synthesize the ether. Ethylsulfuric acid plays its role in case of sulfuric
acid method. I think sodium ethyl sulfate will work too, but it needs to be pre-formed, otherwise this stuff will hydrolize right away.
I did not find any paper that could describe ether synthesis via phosphoric acid even remotely. Phosphate esters are relatively stable and phosphoric
acid is a weak acid. Phosphoric acid on silica can lead to diethyl ether, but I think that on higher conversion rates it will just dehydrate it to
ethylene (phosphate is a bad leaving group).
Ionic resin is capable of promoting the etherification with excellent selectivity, but it either requires some drastic conditions http://qap2.onlinelibrary.wiley.com/doi/10.1002/aic.14497/ab... or is not usable because of polimerisation at higher temperatures.
|
You could be right on the phosphoric acid. I was speaking theoretically.
not sure who you were responding to in your other post but I am not advocating thionyl chloride.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I really want ot correct myself about ethanol-sulfuric acid route being much better than catalytic one. Considering all the information I've gathered
recently, for sulfuric acid route conversion is somewhere at 35-50% per pass, 90-95% of it being diethyl ether when done correctly. That's pretty much
the same numbers as for alumophosphate catalyst on low conversion mode (which is preferential due to higher selectivity). There must be some kind of
equilibrium Et2O + H2O <-> EtOH + Et-Cat which does not allow ether yield to go past the 50% mark, no matter it is heterogenous or homogenous
reaction.
And here's the main thread with sulfuric acid-ethanol discussion and practice: http://www.sciencemadness.org/talk/viewthread.php?tid=9747
[Edited on 13-3-2017 by byko3y]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I've made ether with sulfuric acid a few times. It does produce some disgusting sludges with denatured alcohol but hardly even turns pink with pure
ethanol.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Okay, so I just thought of a dumb idea. Someone explain to me why it won't work.
Potassium pyrosulfate + potassium ethoxide (refluxing ethanol?) >> potassium ethyl sulfate + potassium sulfate
Potassium ethyl sulfate + potassium ethoxide >> diethyl ether (g) + potassium sulfate
K2S2O7
Basically ethoxide attacks pyrosulfate as a nucleophile. I wrote potassium but maybe lithium or some other counterion would be better idfk. Potassium
pyrosulfate is probably not all that soluble in ethanol but i think it would be more soluble than sulfate because disulfurjc acid is a much stronger
acid and salts of superacids are usually pretty soluble
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I think water would be extremely detrimental to the yield. I'm also not sure if it would be possible to carry out the second reaction at a reasonable
rate below the boiling point of ethanol.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I like the idea, but the reaction requires temperature above 100 C (just as a classic reaction does) while alcohol boils at atm. pressure long before
the temperature is reached. Classical synthesis solves the problem via ethylsulfuric acid formation, which is non-volatile but is slowly hydrolyzed.
Working without solvent at 250 C might work, however I have no idea about stability of reagents and intermediates.
Also, synthesis requires not so easily accessible reagents, so I doubt anyone would try it. It might be easier to do a straightforward EtI + NaOEt,
which gives no nasty byproducts.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
You can actually make ether at 70 C, but it's very slow. There's a huge increase in the reaction rate right around 130 C; I think this is due to Gibbs
free energy crossing a threshold permitting a secondary reaction mechanism, but I'm not exactly sure what.
[Edited on 12-6-2017 by JJay]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I was looking for solvents that should be compatible with the reactants and OTC. My best idea so far is mononitrotoluene bp 210 C. I'm not sure if
this is compatible with ethoxide? I'm guessing some of the nitrotoluene will be deprotonated particularly any 2-nitrotoluene (since a mixture of
nitrotoluenes is easiest to make), but not most (ethanol is a stronger acid), and the nitrobenzylsulfonate salt should be inert (to rxn conditions), I
hope? I could see this byproduct causing problems if the mixture is acidified but diethyl ether should distill out and then maybe you recover the
solvent by reduced-pressure distillation?
If I had to guess lithium is the best counterion because of its Lewis acidity.
Other possible OTC solvents for ethoxide include menthol and methylsulfonylmethane.
[Edited on 12-6-2017 by clearly_not_atara]
|
|
|
| Pages:
1
2
3 |