trionic
Harmless

Posts: 9
Registered: 14-6-2013
Member Is Offline
Mood: No Mood
|
|
Diazepine synth options
Hi everyone! I'm a new user here but have been lurking around for a year or so. I'm a budding chemistry student.
Anyway, I'm working on synthesizing a fairly benign pharmaceutical product. I have done alot of research online and found out that there are multiple
ways to do the synth.
If you start out with 2-amino-5-chlorobenzophenone you can either methylate this first by tosylating with tosylCl, Alkylating w. DMSO and finally
hydrolyzing in H2O+H2So4.
Or proceed immediately to cyclocondensation with glycine ethyl ester or as I found out on rhodium; chloroacetyl chloride,hexamine and toluenesulfonic
acid to afford the cyclocondensated product with an amino group that now may be methylated and NH2->N to afford the Diazepam. The one description I
can find on this matter just mentions using DMS, does this mean that tosylation is not required?

I also found an interesting route starting out from 1-Chloro-4-nitrobenzene and benzyl cyanide(phenylacetonitrile) But no description, just an image.
I'm a bit confused here, maybe someone has some pointers on how to go about this the easiest way?
I hope that I'm not being offensive by asking these questions. May I also add that I'm working on a micro-scale for personal use since restrictive
pharmaceutical policies where I'm at forbids me a prescription even though I have a long standing diagnosis of severe anxiety disorder.
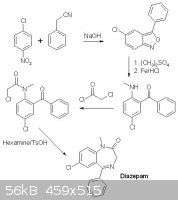
[Edited on 14-6-2013 by trionic]
|
|
|
KrysHalide
Harmless

Posts: 19
Registered: 29-11-2011
Location: France
Member Is Offline
Mood: No Mood
|
|
First of all, that isn't DMSO (dimethylsulfoxide, an aprotic polar solvent) but dimethylsulfate, a very toxic and hazardous alkylating agent.. Do not
get both mixed up, there are completly different!!
The second process just makes your chloro-amino-benzophenone in two first steps, alkylating your chloronitrobenzen, then intramolecular condensation
with benzyl alcohol.. The heterocyle is then reductively opened, offering an aniline (which is alkylated by the DMS) and your benzophenone moity.
From then on, acylation with chloroacetyl chloride (nasty stuff), amination with hexamine (sommelet reaction) and intramolecular condensation..
Don't want to discourage you, but this seems like a complicated synthesis for someone with only basic knowledge and experience.. I would first try out
easier synths, because handliung DMS and ClAcCl is not begginer-friendly..
Never regret thy fall,
O Icarus of the fearless flight
For the greatest tragedy of them all
Is never to feel the burning light.
Oscar Wilde
|
|
|
trionic
Harmless

Posts: 9
Registered: 14-6-2013
Member Is Offline
Mood: No Mood
|
|
Hi!
Thank you for the pointers! I'll make sure to not mix up the dmso. I have already done some fairly complicated synth's like
2-methyl-3-(2-methylphenyl)-4-(3H)-quinazolinone from Anthranilic acid with excellent yield, so I think I might pull this off.. I know about the
ClAcCl, I already have some and I will do this in the fume hood. Don't worry!  The Sulfate do worry me though, I won't do it... I'll either have to methylate with a more safe agent or try to find some pre-methylated 2-a-5cbp
The Sulfate do worry me though, I won't do it... I'll either have to methylate with a more safe agent or try to find some pre-methylated 2-a-5cbp
I can get 2-amino-5-chlorobenzophenone or phenylacetonitrile etc. from my local supplier. But not the methylated 2-methylamino so that's why I'm
asking about the best way to start this synth...
[Edited on 14-6-2013 by trionic]
[Edited on 14-6-2013 by trionic]
|
|
|
simba
Hazard to Others
  
Posts: 175
Registered: 20-5-2011
Member Is Offline
Mood: No Mood
|
|
You can do the methylation with methyl iodide instead, which is pretty nasty also, but much less dimethyl sulfate.
|
|
|
KrysHalide
Harmless

Posts: 19
Registered: 29-11-2011
Location: France
Member Is Offline
Mood: No Mood
|
|
You will not be able to directly methylate the primary amine without giving tertiary and quaternary amines.. Check out a thread about "monomethylation
of primary amines" by klute, protection via benzaldehyde-imine will offert pur mono-methyl amines.
Sulfonates would be a safer option here, methyl tosylate (cf Klute), or methyl methane sulfonate (cf Ullmann, in the sulfuric duff thread) are easily
obtainable and pretty efficient.
Si if I were you, I would react your amino-chlorobenzophenone with the glycinate est, using cat. TsOH in toluene,, and a dean stark for best results,
then add TsOMe in toluene, gently reflux, and workup., that seems like it.
But if you really plan on using your chloracetyl chloride, add it diluted in DCM to your mono-methylated substrate in DCM, don't bother purifying
after the mono-methylation, a simple A/B gives a very clean product) at 0°C with an equivalent of triethylamine, wash off the EtN.HCl when done, and
proceed to cyclization (again, a dean stark could be usefull)
[Edited on 14-6-2013 by KrysHalide]
Never regret thy fall,
O Icarus of the fearless flight
For the greatest tragedy of them all
Is never to feel the burning light.
Oscar Wilde
|
|
|
Nicodem
|
Thread Moved
14-6-2013 at 11:51 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by trionic  | | The one description I can find on this matter just mentions using DMS, does this mean that tosylation is not required? |
Think about it. If you N-tosylate it, then there will be no position left to N-methylate it.
Quote: Originally posted by simba  | | You can do the methylation with methyl iodide instead, which is pretty nasty also, but much less dimethyl sulfate. |
Do you have a reference in support of this? What is the N-methylation vs. C-methylation selectivity?
Quote: Originally posted by KrysHalide  | | You will not be able to directly methylate the primary amine without giving tertiary and quaternary amines.. Check out a thread about "monomethylation
of primary amines" by klute, protection via benzaldehyde-imine will offert pur mono-methyl amines. |
Are there any examples of this strategy being applied to anilines? Imines are poorly nucleophilic and the imines derived from the aromatic amines are
even so very much less nucleophilic.
| Quote: | | Si if I were you, I would react your amino-chlorobenzophenone with the glycinate est, using cat. TsOH in toluene,, and a dean stark for best results,
then add TsOMe in toluene, gently reflux, and workup., that seems like it. |
Anilides don't get N-methylated so easily. They generally need to be deprotonated first. Some aliphatic amides do get slowly methylated with in their
neutral form with methyl sulfonates or dimethyl sulfate, but in such a case the O-methylated product is the kinetic product obtained (e.g., like in
the reaction of DMF with dimethyl sulfate).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
KrysHalide
Harmless

Posts: 19
Registered: 29-11-2011
Location: France
Member Is Offline
Mood: No Mood
|
|
Good point, I had somehow abstracted the fact that it was an aromatic amine.. My comments do not apply here then...
Never regret thy fall,
O Icarus of the fearless flight
For the greatest tragedy of them all
Is never to feel the burning light.
Oscar Wilde
|
|
|
simba
Hazard to Others
  
Posts: 175
Registered: 20-5-2011
Member Is Offline
Mood: No Mood
|
|
http://www.scielo.br/scielo.php?pid=s0103-50531998000400010&...
|
|
|
trionic
Harmless

Posts: 9
Registered: 14-6-2013
Member Is Offline
Mood: No Mood
|
|
All this seems a bit tricky.. I'm now consedering making a certain nitrated compund instead. This way I don't have to consider methylation since the
diazepines with the n2o group has an amino group instead of methyl group. The missing necesseray precursor is available for me and everyting else like
Chloroacetyl chlorode,hexamine, solvents etc. i got already.. I'm gonna order some nitrated precursors instead now. Wish me luck!
|
|
|
ClandistineChemist
Harmless

Posts: 1
Registered: 26-6-2013
Location: Indonesia
Member Is Offline
Mood: Experimental
|
|
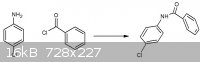
The first question is: HOW to make 2-amino-5-chlorobenzophenone?
Side reaction is posted in attachment, so how do you think to prevent amide formation by performing a FC acylation?
You can claim everything here on the internet but without peer - reviewed articles it isn't true in science.
__________________________________________________
The second question is what is the reason that p-chloroaniline reacts in a alkaline environment to form the product in scheme 2?
If you have a source and don't like to post it down here, just send a PM.
Off - topic
Diazepam is OTC here in Indonesia. My opinion is if Valium is not legal, why is alcohol then legal?
Shit happens !!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Are we talking about diazepine or diazepam? Either way the benzodiazepines are among the nastiest sedatives ever invented. Be kind to yourself and
make some quaalude. An undergraduate ought to be able to handle that synth. Or how about a glass of warm rice wine?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Number 9
Harmless

Posts: 34
Registered: 23-3-2012
Location: Grajagan
Member Is Offline
Mood: ingin tahu semua
|
|
As biochemist I don't recommend methaqualone or barbituates either. It can cause not only dependence but also the dose is pretty high, OD is a big
risk.
Withdrawals are dangerous when the subject is diazepam with the diazepan ring. In rare cases (like some Vietnam veteran's) the DOSE is simply too high
and in that particular case you get strong withdrawal symptoms that are dangerous (first 10 days): spasm, shaky, headache (3 days), sweating, dilated
pupils and the chance of getting a seizure. These symptoms go over and most patients are fully recovered after 1 or 2 years. Please read the Ashton
manual.
Sternbach, L. (inventor of Librium) did perform the reaction in the scheme above by a reaction between p-chloro aniline and benzoyl chloride in the
presence of ZnCl2 as Lewis acid. He use diluted sulphuric acid in acetic acid but I don't know why?
Difficult synthesis however, you need a vacuumpump to prepare p-chloro aniline and you must prepare benzoyl chloride outside! Fuming HCl escapes very
rapidly by slightly heating. You can buy a car battery to fix the job outside.
Also, use a excess of benzoyl chloride for that FC acylation. Reaction time: 8-9 hours.
Take care!
|
|
|
trionic
Harmless

Posts: 9
Registered: 14-6-2013
Member Is Offline
Mood: No Mood
|
|
Sorry guys, I forgot about this thread!
TO answer some questions... I have been addicted to diazepam/benzoes and had two seizures during withdrawal. But I don't touch that stuff any more
since my Doctor gave me a nice prescription for LYRICA (pregabalin) which is also addictive, but hey,, I have REALLY bad anxiety and diagnosis from
several psychologiststs!
Anyway, I Have synthesized Methaqualone and enjoyed it but the dosage is too high I mean 300mg for one dose? I would prefer to synth something in the
1-2mg dosage range  And you who suggest Alcohol!? NO way i'm
drinking solvents, mmkay! And you who suggest Alcohol!? NO way i'm
drinking solvents, mmkay!
Anyway you can see my new thread that I'm going to make clonazepam just because I still have all the chems and it would be a shame to let them just
sit on the shelf plus, my mad supplier got me some sickly nice precursors.
NEW THREAD
https://www.sciencemadness.org/whisper/viewthread.php?tid=30...
|
|
|