| Pages:
1
..
10
11
12
13
14
..
38 |
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by DJF90  | | Same thing goes for a sep funnel. Always pour your reaction mix from the flask to the sep funnel through a plastic funnel with a narrow enough
aperture to catch the stirbar - result: no breakages upon shaking. |
You can also just use a hard drive magnet to stick the stirbar to the flask wall when you pour.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
make yourself one of these:
http://www.sciencemadness.org/talk/viewthread.php?tid=13156&...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Link not works...
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The link works for me. It takes me to a thread, where upon scrolling up I find my post with the picture of my stir bar retriever. If you still can't
find it let me know and I will quote the whole post in response.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by Magpie  | | The link works for me. It takes me to a thread, where upon scrolling up I find my post with the picture of my stir bar retriever. If you still can't
find it let me know and I will quote the whole post in response. |
The link still doesn't work (for me at least). It takes me to a default page "the item you have requested cannot be found" type thing.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 857
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
It works for me. What browser are you using? I'm using Opera v. 11.50.
This just in: 95,5 % of the world population lives outside the USA
You should really listen to ABBAPlease drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Magpie  | Finding that I often wish I had one of these but am not willing to pay the $20 + postage, I made one of my own. Here's how:
* Buy 2 feet of 0.17"ID polyethylene tubing ($0.34)
* Buy one 3 foot piece of 1/16" steel rod (~$2)
* Buy one 3/16"OD x 1/2" long cylindrical neodymium magnet ($0.27) (Note 1)
1.Heat about 3/4" of one end of an 18" piece of poly tubing in boiling water to soften it, then force fit the magnet into it, leaving about 1/4" of
the end of the tube open.
2. Fuse this end of the poly tube by heating it on a stove, working the end to a seal. Try not to overly heat the magnet as it will lose its
magnetism if heated to 80C. (Note 2.)
3. Cut the steel rod to fit in the poly tube leaving about 1/2" of the tube end open.
4. Fuse the open end of the poly tube as before.
Notes
1. The economics are hurt here if you have to pay postage on the magnet like I did. This came to about $5.
2. It might be better to buy an undersize magnet, say 1/8"OD instead. That way you could slide the magnet in later after fusing the end to a seal,
and wouldn't have to risk heating the magnet. These Nd magnets are tremendously strong.
[Edited on 23-1-2010 by Magpie] |

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I'm using Safari version 5.0.5.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Speaking about bad days with glassware, I was messing around, minding my own business when this happened:

I know soda lime glass crack under the slightest temperatire changes, but I ramped up the heat extremely slowly, bringing the water to a boil in 40
minutes. The whole bottom usually doesn't blow out when I'm this careful, it's the first time.
I guess the glass had some internal stress to begin with, oh well bad luck I guess.
I gotta get me som'o those polarizers next time
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
That doesn't look like glassware. It looks like a pickle jar. They are not known for their heat resistance.
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
Indeed, I use plain old mason jars to store chemicals, mix them or react them if they are only mildly exothermic, but I have stopped using them to
heat/boil/react stuff in them if it might be a bit too intense. I have had jars fracture simply because I have removed them off the hotplate or water
bath.
It is safer to boil or heat stuff in a proper borosilicate vessel, using mason jars or mayonnaise pots to heat/boil/react stuff is at your own risk,
and if the vessel breaks, you lose your chemical you've worked on and run the risk of ruining your heating device at the very least.
Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
The other day I had a small incident in the lab. I performed the gas test for hydrogen on one reaction that was running, which consists of holding a
lit splint to the test tube and listening for the pop. After the test, I blew out the splint and went to snuff out the glowing end on a piece of
filter paper lieing nearby. I've done this many times without incident, but this particular paper immediately started crackling and burst into flames!
I ran it over to the sink to extinguish it, and luckily nothing was damaged. The filter paper had been used earlier to filter nickel hydroxide from a
solution containing potassium nitrate, and so had some residue on it. I think the KNO3 was the culprit.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Yes, but believe it or not, I tested one of these out one time to find the limits of these jars, and I was able to bring water to a boil (quickly) and
sustain a boil for more than 20 minutes without it breaking.
I suppose you can get lucky sometimes. For the record, I never boil anything of considerable value in jam jars. In this case, I was extracting urea
from fertiliser. I was boiling about 100mL of water mixed with approximately the same volume of fertiliser and ramped up the heat really REALLY
slowly. I've had some instances where the glass gave a warning, cracked but didn't break completely. I was hoping for that, but instead the whole
bottom blew off without warning.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Halcyon
Harmless

Posts: 42
Registered: 5-10-2010
Member Is Offline
Mood: No Mood
|
|
In my first high-school science class, the teacher made a huge point of how expensive the measuring cylinders were and how careful we had to be. One
dude knocked over a 250ml, I turned to point and laugh, and stole his thunder by knocking over a 500ml one.
FML, that day.
|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i hate glass and glass hates me back! i was cleaning a round bottom flask after a distillation when this happen...
 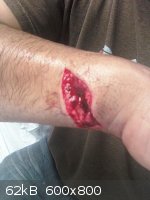
bad day indeed...
[Edited on 15-2-2012 by neptunium]
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
   
Hope to God it doesn't get infected.
[Edited on 2-15-2012 by White Yeti]
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
this was last year in June ...went to the hospital no infection thanks! i am fine now...but it was a bloody mess
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Broke my 14/20 claisen adapter. I blame the Teflon tape I used. I have just read about it's problems with thermal expansion. As soon as the
thermometer read >100°C it popped right off.

EDIT;
Damn neptunium, that's nasty.
[Edited on 27-2-2012 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Holy crap, neptunium! :O
Any severed nerves? Any cool looking scars? 
These kinds of photos are actually good to look at - one becomes aware of the dangers of handling glassware. I think we all tend to ignore that fact
because glassware is smooth and cool to play with. This is an eye opener for some, a good reminder for others.
Bot0nist, was that one layer of tape or more? Was the tape covering the lower or the upper part of the joint?
[Edited on 27-2-2012 by Endimion17]
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Quote: Originally posted by Endimion17  |
Bot0nist, was that one layer of tape or more? Was the tape covering the lower or the upper part of the joint?
[Edited on 27-2-2012 by Endimion17] |
Only the top <sup>1</sup>/<sub>4</sub> was wrapped. One and a half layers.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
That's a shame Bot0nist, I broke a 250ml 24/40 flask a few days back--I flipped because it was totally my fault. The next day I got my glassware
kit--and it was all a-ok 
BOLD
|
|
|
inspector071
Hazard to Self
 
Posts: 69
Registered: 20-2-2012
Member Is Offline
Mood: No Mood
|
|
I broke a 500 mL retort after my 2nd nitric acid distillation with the thing about a week ago. There was a solid cake of potassium sulfate left and I
was trying to break it up so I could get back to distilling some more nitric acid. The cake of course thumped up against the glass, which I
over-estimated the strength of, and promptly broke. Next time, I'll be more patient and let the potassium sulfate dissolve in hot water. Looking back,
I would have been better off just buying a 500 mL distillation setup.
|
|
|
Mirage
Harmless

Posts: 49
Registered: 26-1-2012
Location: Canada
Member Is Offline
Mood: Superheated
|
|
I was just doing a simple steam distillation, and as I removed the hotplate from underneath the erlenmeyer flask, the whole setup fell over! I had my
ring stand twisted the wrong way! Everything proceeded to fall to the damn tile floor, and smash. My steam distillation head with vacuum takeoff
smashed, my heavy wall Erlenmeyer, and the bottom portion of my fancy coil condenser. NOOOO! Everything was Chemglass! Expensive! Luckily my receiving
flask was fine as well as my vigreux.
Mirage
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Damn, that sucks. Happened to me once too, but I had a liter hot oil bath hit the floor with my glass! What a mess.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by neptunium  | i hate glass and glass hates me back! i was cleaning a round bottom flask after a distillation when this happen...
bad day indeed...
[Edited on 15-2-2012 by neptunium] |
.....Holy fuck dude.... and you stopped to take a picture, are you serious?
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
| Pages:
1
..
10
11
12
13
14
..
38 |