| Pages:
1
..
66
67
68
69
70
..
103 |
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
What is the maximum temperature you can safely reach in a round-bottom flask in an air bath on a regular ceramic hot plate like the PC-351?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Not sure about air baths, but I've seen heating mantles get hot enough to soften the pyrex glass. About 350 C, IIRC.
Do you mean safe for the glass, or safe for the hot plate?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Safe for the hot plate... I have a heating mantle, but it is too large for the flask, and I've had awful luck with sand baths.
I guess maybe I'll just break out my propane burner and wire gauze....
[Edited on 31-1-2017 by JJay]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I think pretty much everyone on here knows by now that crystals often have water in the crystal structure. For example, the sodium carbonate you buy
at the grocery store usually isn't pure sodium carbonate; it's sodium carbonate monohydrate, with one mole of water molecule per mole of sodium
carbonate. Copper sulfate sold as root killer is copper sulfate pentahydrate, with five moles of water per mole of copper sulfate. If a crystalline
substance contains half a mole of water per mole, it's a hemihydrate. And so on....
Substances crystallized from methanol or ethanol often have methanol or ethanol in the crystal lattice. I have never been taught or read any clear
rules on how to name such crystals, though... does anyone know what to call a crystal of a substance that contains one mole of methanol per mole of
substance, for example?
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
Question
I made some aluminum Chloride by dissolving aluminum in HCl. Well there was a blackish brownish suspension left so I preformed a Vacuum Filtration to
remove all of this. When I tried to clean out the sintered vacuum funnel it was super resistant. Couldn't even get pirranah solution to clean it out.
What is this stuff and how do I clean it out?
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 184
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
Maybe try HCl since it is what you originally used. Maybe use strong bleach?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by JJay  | I think pretty much everyone on here knows by now that crystals often have water in the crystal structure. For example, the sodium carbonate you buy
at the grocery store usually isn't pure sodium carbonate; it's sodium carbonate monohydrate, with one mole of water molecule per mole of sodium
carbonate. Copper sulfate sold as root killer is copper sulfate pentahydrate, with five moles of water per mole of copper sulfate. If a crystalline
substance contains half a mole of water per mole, it's a hemihydrate. And so on....
Substances crystallized from methanol or ethanol often have methanol or ethanol in the crystal lattice. I have never been taught or read any clear
rules on how to name such crystals, though... does anyone know what to call a crystal of a substance that contains one mole of methanol per mole of
substance, for example? |
mono-methanol-ate
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Are you sure? That seems reasonable, but the most common use of methanolate seems to be to denote methoxide....
Edit: Nomenclature in this area is confusing, but you are correct.
[Edited on 1-2-2017 by JJay]
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
Why would bleach work???
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Molbase?
I just stumbled across a website called Molbase. They appear to be a service which specifically finds chemical suppliers. They have places in North America, India and China, but are
based primarily in China. Some prices are intriguing, like this one; 1kg lidocaine for $30. I though making denatonium benzoate like NileRed would be cool, and this would be an amazing price. Even if there
was way overpriced shipping it would still be amazing. Does anyone have experience with this website? Is it a legiamite thing?
Thanks
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Quote: Originally posted by TheNerdyFarmer  | | I made some aluminum Chloride by dissolving aluminum in HCl. Well there was a blackish brownish suspension left so I preformed a Vacuum Filtration to
remove all of this. When I tried to clean out the sintered vacuum funnel it was super resistant. Couldn't even get pirranah solution to clean it out.
What is this stuff and how do I clean it out? |
Where did the aluminum come from? The black-brown stuff is probably silicon. Cast and rolled aluminum alloys tend to contain significant amounts of
silicon from the casting process. If this is the case, I don't think that anything beside HF would dissolve it. Remember to use filter paper if you
try again.
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
I have an idea. If I pass chlorine gas through the funnel, shouldn't the chlorine react with the silicon to form silicon tetrachloride? please leave
some input on this if you can. BTW I know that silicon tetrachloride is super dangerous but I don't have HF which is probably more dangerous.
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Chlorine might work, but I am not sure. That's a little out of my realm of expertise. By the way, hydrofluoric acid would probably be a bad choice to
clean a frit anyway, since the frit would also be dissolved to some extent.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by TheNerdyFarmer  | | I have an idea. If I pass chlorine gas through the funnel, shouldn't the chlorine react with the silicon to form silicon tetrachloride? please leave
some input on this if you can. BTW I know that silicon tetrachloride is super dangerous but I don't have HF which is probably more dangerous.
|
I'm not sure if that would work near room temperature, I've never seen such a reaction reported. On a more positive note, if you can't find anything
to dissolve the silicon and remove it, it's likely that the only consequence to the frit will be cosmetic.
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
So if I leave it like it is, it won't bother anything?
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Erlenmeyer vs RBF
I usually use an air bath for heating RBFs because it is simple, although it suffers in efficiency. For better heating, larger quantities or high
temperatures I use a water or oil bath. Twice now I have spilled the oil bath which leads to a huge mess and oil all over the bench and hotplate. I am
now contemplating buying a bunch of jointed erlenmeyers. There would be more heat transfer to the flask and better stirring. For simple distillations
and reflux I don't usually need more than one neck, and addition can be through the condenser if needed. Is there any reason why RBFs are so much more
popular than erlenmeyers? Are they superior? Nurdrage seems to use them a lot so I think I might get some.
Any input is appreciated, thanks.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
I use Erlenmeyer flasks for vacuum filtration with little worry of implosion
because the Erlenmeyer'sf or vacuum are thick and/or shaped for vacuum,
thick glass experiences much more stress than thin glass whith rapid temperature changes or steep temperature gradients,
I do not fancy an Erlenmeyer with heat, vacuum, and a flat bottom.
but the overall cost of going to a vacuum system makes the cost of a few flasks insignificant.
I like the stability and ease of heating and ground neck of Erlenmeyers.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I hardly ever use Erlenmeyers, but I do like how they can be heated directly on a hotplate and stand up on their own.
I recently started using table salt baths. They take a while to heat up and would make a mess if spilled but are much less messy than oil baths and
can reach higher temperatures. I think heating blocks would be even better, but they are expensive.
Usually, if doing a reaction that requires a ground glass joint, I would want the Erlenmeyer to be clamped to a stand anyway, so their ability to
stand on their own is less of an asset than one would think... and usually I just use beakers for reactions that don't require ground glass joints, so
I hardly ever use Erlenmeyers.
For precise temperature control, it's really hard to beat a multi-neck round-bottom flask in an oil bath.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
How to regulate water flow ?
For the next phase of my distillation experiments I will need to regulate the flow of cooling water through various condensers,
using water at pressure from the domestic supply.
When I recently ran a few distilations I had to re-adjust the garden tap several times to maintain a specific flowrate,
(possibly due to the rubber washer slowly relaxing after being compressed for so long),
I also noticed random changes in water pressure,
(probably due to varying local demand)
What is a cheap and easy way to get a constant flowrate from a varying pressure water supply ?
Worst case ... I'll use a header tank.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  |
What is a cheap and easy way to get a constant flowrate from a varying pressure water supply ?
Worst case ... I'll use a header tank. |
Pressure reducing regulators are available. For example Screwfix has them for a round £25. But becarful as not all of them can be adjustable down to
zero pressure. I got one in a B&Q sale. It does go down to zero pressure but its regulation wrt to flow rate is poor but if its only suppling a
continuous flow that may not matter.
If your into skip diving you can find old header tanks complete with the ball cock and fittings in them as modern boiler replacements are direct
feeds.
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Capacity of soxhlet
I was thinking of buying this Soxhlet extractor, but I was wondering about the capacity. It says 250ml, so I would think that the volume in the extractor is 250cc.
However, based on the picture I would say it looks like the extractor could hold 125ml.
Does the 250ml refer to the volume of the chamber or the amount of solvent to be used with it? Perhaps the picture is of a 125ml one or my estimates
are off by a factor of two.
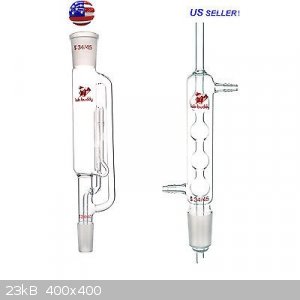
Edit: That was a stupid question, it says right in the listing that I has a 75ml capacity, I should really pay more attention. In that case the
"250ml" likely refers to the size flask meant to be used with it.
If anyone else was confused like me the soxhlet size is not actually its volume, the actual capacity of the soxhlet is much less.
[Edited on 2/8/2017 by Geocachmaster]
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
I had a brilliant but simple idea,
I will supply cooling water for my condensers via a tube siphoning from a toilet cistern
... that is already available, my upstairs bathroom.
I'll just have to put a notice
PLEASE DO NOT FLUSH ... DISTILLATION IN PROGRESS
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by Geocachmaster  | I usually use an air bath for heating RBFs because it is simple, although it suffers in efficiency. For better heating, larger quantities or high
temperatures I use a water or oil bath. Twice now I have spilled the oil bath which leads to a huge mess and oil all over the bench and hotplate. I am
now contemplating buying a bunch of jointed erlenmeyers. There would be more heat transfer to the flask and better stirring. For simple distillations
and reflux I don't usually need more than one neck, and addition can be through the condenser if needed. Is there any reason why RBFs are so much more
popular than erlenmeyers? Are they superior? Nurdrage seems to use them a lot so I think I might get some.
Any input is appreciated, thanks. |
just wrap a foil skirt around your RBF, encapsulating the entire surface of the hot plate in with the flask. The increase in heating efficiency is
massive, perhaps better than direct contact. Similarly I place a wall of foil around large beakers or erlenmeyers in contact with the hot plate to
reduce heat loss from the sides of the container.
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
Hello, I was going to make sodium nitrite via heating sodium nitrite with carbon in the presence of calcium hydroxide. I got this off of the SM wiki
and it did not specify what temperature the reaction was to be heated to. If some of you have tried this method please do let me know if it is worth
doing. 
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
Question
What is calcium hydroxide soluble in. And for future reference, how to do find the solubility of chems in organic solvents and alcohols ect. I can
never find it online.
[Edited on 10-2-2017 by TheNerdyFarmer]
|
|
|
| Pages:
1
..
66
67
68
69
70
..
103 |