| Pages:
1
..
92
93
94
95
96
..
103 |
paulll
Hazard to Self
 
Posts: 99
Registered: 1-5-2018
Member Is Offline
Mood: It's fine. Really.
|
|
Good to know, ty.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
My NaBr is slightly halogeny...perhaps from the original crystallization or reaction with the container at some point. Our noses are very sensitive
analytical instruments sometimes...e.g. a super low concentration of IPA smells pleasant and is unrecognizable as such.
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
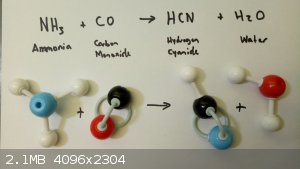
Hypotherical hydrogen cyanide synthesis
I just composed a problem for my students – calculation of enthalpy of reaction from tables of average bond energies.
Seemed like an ok example of the concept. But is there a feasible reaction schema that produces HCN from ammonia and carbon monoxide?
Just curious.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I don't think so, but that's a good example.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Carbon monoxide is a better reducing agent than it is an oxidizing agent, and ammonia needs to be oxidized to give cyanide.
Reflux condenser?? I barely know her!
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Quote: Originally posted by njl  | | Carbon monoxide is a better reducing agent than it is an oxidizing agent, and ammonia needs to be oxidized to give cyanide. |
Should have thought of that.
(Shhh. I won't tell the students. It will ruin the illustration.)
|
|
|
paulll
Hazard to Self
 
Posts: 99
Registered: 1-5-2018
Member Is Offline
Mood: It's fine. Really.
|
|
Too late to offer bonus marks for balancing the equation?
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
It is an exercise to introduce a concept and not an assessment. So, no marks. What is required is clarity, and a situation that is non-trivial to
give purpose to the exercise and so that the required concepts are repeated a few times. This meets those criteria.
|
|
|
scienceboi
Harmless

Posts: 31
Registered: 12-9-2019
Location: California
Member Is Offline
Mood: Curious
|
|
Question
Can metals become plasmas? And how easy or useful is it to make them plasma?
I was just looking up sputter deposition for silicon wafers and it got me thinking.
"Read not to contradict and confute; nor to believe and take for granted; nor to find talk and discourse; but to weigh and consider." -Francis Bacon
|
|
|
Hoffit
Harmless

Posts: 21
Registered: 12-8-2019
Member Is Offline
Mood: Excited
|
|
Applied science has some nice videos about sputtering like this one https://www.youtube.com/watch?v=9OEz_e9C4KM
|
|
|
pneumatician
Hazard to Others
  
Posts: 409
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
What is the ideal acid nitric concentration for dissolving Iron? 30%? 20%? 10%?...
Pure of around 99,9x% and "normal" Iron filing?
[Edited on 30-5-2021 by pneumatician]
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I would say it depends on what you're after. If it's reaction speed it would probably be strong acid, but that tends to produce lots of NO and NO2.
For a clean reaction with little loss I would keep the concentration below 20%.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
B(a)P
International Hazard
    
Posts: 1114
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Concentrated nitric acid will passivate iron.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by j_sum1  | I just composed a problem for my students – calculation of enthalpy of reaction from tables of average bond energies.
Seemed like an ok example of the concept. But is there a feasible reaction schema that produces HCN from ammonia and carbon monoxide?
|
If you factor entropy into your calculations I'm betting you'll find that delta-G is always positive for the forward reaction (forbidden). But IIRC
you can do it in two steps, by making formamide first and using a selective catalyst second.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
pneumatician
Hazard to Others
  
Posts: 409
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
what is the Pepy's apparatus???
|
|
|
Belowzero
Hazard to Others
  
Posts: 173
Registered: 6-5-2020
Location: Member Is Offline
Member Is Offline
|
|
Will acrylate or plexiglass resist ~30% nitric acid for long periods of time at room temperature?
Or PVC?
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
https://en.wikipedia.org/wiki/William_Haseldine_Pepys
May be this will help? You didn't mention in what activity the apparatus might be used in.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Carboxylic acids can be activated by formation of mixed anhydrides. Could the acid instead be reacted with isocyanate to yield a carbamate (mixed
carbamate anhydride)?
Reflux condenser?? I barely know her!
|
|
|
pneumatician
Hazard to Others
  
Posts: 409
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
someine use one of this? ocerall qlty?? leaks in joint???
https://www.ebay.de/itm/152510588408?ssPageName=STRK%3AMEBID...
|
|
|
pneumatician
Hazard to Others
  
Posts: 409
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
near 0 info in archive and gbooks! :-?
|
|
|
SHADYCHASE54
Hazard to Others
  
Posts: 150
Registered: 16-12-2010
Location: CaNaDay!
Member Is Offline
Mood: No Mood
|
|
Hello fellow S.M'ers,
I am wondering if I were to make hypophosphorus acid with it's Na salt and 32% HCl; then flash distill the resulting solution under strong vacuum to
remove H2O, precipitate most of the NaCl while keeping the acid's boiling point below its d-comp. What would be, after diluting the acid to 10-15
percent of course, the most suitable exchange resin(s') to remove the Na and Cl impurities? Would reacting the diluted solution first with H+ strong
acid cation exchange resin convert the remaining NaCl to HCl? Furthermore I am wondering if I were then to react the resulting solution with a strong
anion exchange resin in the Cl- form would this remove the Cl from the HCl impurity thus liberating the hydrogen (g)?
I have no idea if this would work past making the acid solution. Anyone with experience or knowledge with or about exchange resins, who is willing to
point me in the right direction, I would really appreciate it.
2 side notes: 1 yes I am aware of the danger of this acid's decomp. products. 2 the hypo is not for any illicit use, truthfully I haven't even decided
what I intend to reduce yet? As the real experiment is in reaction conditions and comparing those to similar reducing agents. I am also interested in
comparing it's reducing capacity under various reaction conditions. Such as simple reflux, microwave irradiation and under pressure. (In an actual
pressure bomb). There I'm done.
Any help would be appreciated.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
TCCA + EtOH = Chloroform?
I have performed the classic acetone + hypochlorite haloform reaction a couple of times,
I would like to try TCCA instead of hypochlorite,
will it make much difference if I use EtOH (85% or more) instead of acetone.
(I have lots of (pool grade, 90%)TCCA and diy EtOH)
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
BauArf56
Hazard to Self
 
Posts: 68
Registered: 22-8-2019
Location: between the moon and the sun
Member Is Offline
Mood: energetic
|
|
is it possible to isolate any chlorinated compound by burning propane in chlorine?
Once i wanted to see how does propane (or butane) burn in chlorine gas, so i lighted up the blowtorch inside a bottle filled with chlorine. It burned
with a bigger flame and it was quite luminous too. After a few seconds of burning inside the bottle there was no smell of chlorine at all, just the
typical sweet smell of chlorinated compounds. Can anyone explain which compounds form and if is it possible to isolate them as liquids? Thanks
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
I have not bought that particular one, but they are commonly used in labs. For large quantities they have significant advantages in filling and
cleaning. The top portion generally uses a standard 100mm opening, you can buy various sealing rings but the ground glass joints should meet as well
as a standard vacuum desiccator.
You can buy various tops for the flask:
https://www.ebay.com/sch/i.html?_from=R40&_trksid=p23220...
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Quote: Originally posted by Sulaiman  | I have performed the classic acetone + hypochlorite haloform reaction a couple of times,
I would like to try TCCA instead of hypochlorite,
will it make much difference if I use EtOH (85% or more) instead of acetone.
(I have lots of (pool grade, 90%)TCCA and diy EtOH) |
TCCA will not work by itself. You will have to add a soluble hydroxide base. And you will need extra as the cyanuric acid will need to be
neutralized to form the final product.
There is actually a thread on TCCA.
http://www.sciencemadness.org/talk/viewthread.php?tid=5686
Ethanol will work, It has an alpha carbon.
Ethanol was used in the first haloform reaction.
Since you normally add water in the form of bleach or to dissolve the calcium hypochlorite, the water content won't matter much.
However, ethanol and chloroform give an azetrope so you need to be sure your ethanol totally reacts. Acetone has a similar issue with separation.
|
|
|
| Pages:
1
..
92
93
94
95
96
..
103 |