| Pages:
1
..
96
97
98
99
100
..
103 |
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
The salt is deliquescent so I GUESS that some sort of dessicator is required.
Edit : just checked.. <30% RH to dessicate.
Also, decomposes above 55C
[Edited on 22-6-2022 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
thank you , but tell me this in many of the articles read and videos viewed , the topic of the reaction being anhydrous only shows up when using NaH
as a base but otherewise nothing in the use of the bases methoxide or ethoxide....why is that? Is it something one should know from the start
.....I had my sample of 4-amina acetophenone analysed , and it turns out to be benzocaine and benzoic acid.....is it that the amine reacted with the
enol or the base....quite confused ....I'm currently on test #13 and the same results even though i introduce the ethyl ethoxide last slowly with an
excess of benzocaine ....but the results are the same once i add the HCl to keep the reaction to the right.....then heated to decarboxylate.......any
hints , and yes i've run the reaction with anhydrous reagents, but the results seems to be the same.......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by Sulaiman  |
The salt is deliquescent so I GUESS that some sort of dessicator is required.
Edit : just checked.. <30% RH to dessicate.
Also, decomposes above 55C
[Edited on 22-6-2022 by Sulaiman] |
Where are you getting this? I've never heard of any special drying apparatus being necessary. Wikipedia gives a melting point of 75C, a dehydration
point of 130C, and a decomposition point of 220C so I don't feel too bad about heating the aqueous solutions.
I poked and prodded the syrup, dripped ethanol and acetone on the surface, smeared a thin layer on the side of the beaker, left a toothpick in...
nothing. I would have thrown it out except I got lazy cleaning and then after many weeks of sitting I thought I saw mold in it... no, the syrup was
crystallizing in a couple points! Within a day or so it was solid throughout. I saved a bit, redissolved the crystallized mass, and then used the
residue as a seed. I now have a jumble of fat needles, not really the blocks I expect but I'm going to use them to reseed another round and see how
that goes.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It's on Wikipedia.
| Quote: | | Rochelle salt crystals will begin to dehydrate when the relative humidity drops to about 30 per cent and will begin to dissolve at relative humidities
above 84 per cent. |
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
"dehydrate" in that passage refers to the loss of water of crystallization, not crystallization from solution.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
The benzylic bromination of 2-methylnaphthalene, using NBS, is ideally done in presence of a radical initiator, benzoyl peroxide is usually used for
that purpose.
With such a radical initiator, I could use a number of solvents, as the benzylic bromination will in that case be the main- and not side reaction.
Tried it once without, solventless, 20h in a melt, and the yield was(side reaction aromatic bromination) just a third of the theoretical yield.
So, here is the question: can I use the 2,4-dichlorobenzoylperoxide(50% under some silicon oil, because explosive  ) as alternative radical initiator? ) as alternative radical initiator?
I expect the answer to be "of course", but I figured it won't hurt to ask.
verrückt und wissenschaftlich
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I'm making 5-formyl vanillyl and after reacting vanillyl and hexamine, and adding HCl I dumped the reaction on ice. The reaction contains 55 grams
water, 55 grams acetic acid, 50 ml 30% HCl, and about 50 grams organics. I dumped this hot mixture on 400 grams of ice of approximately 0 degrees.
Now what I didn't expect was this mixture to go below 0 degrees, but it went all the way down to -7 degrees. Why is that? What endothermic process is
going on here?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by Tsjerk  | I'm making 5-formyl vanillyl and after reacting vanillyl and hexamine, and adding HCl I dumped the reaction on ice. The reaction contains 55 grams
water, 55 grams acetic acid, 50 ml 30% HCl, and about 50 grams organics. I dumped this hot mixture on 400 grams of ice of approximately 0 degrees.
Now what I didn't expect was this mixture to go below 0 degrees, but it went all the way down to -7 degrees. Why is that? What endothermic process is
going on here? |
Ice melting is endothermic. Because you've got a lot of acid, that lowers the melting point just like salt would.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
when nitrosyl chloride reacts with limonene, why does it add only to the cyclohexene double bond, and not to the 2-propene side chain?
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | | ... adding HCl I dumped the reaction on ice... what I didn't expect was this mixture to go below 0 degrees, but it went all the way down to -7
degrees. Why is that? What endothermic process is going on here? |
IIRC the mixture of ice and concentrated
HCl solution produces quite low temperatures, you diluted HCl in other reagents but anyway you still observed the endothermic process
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Quote: Originally posted by mayko  | | when nitrosyl chloride reacts with limonene, why does it add only to the cyclohexene double bond, and not to the 2-propene side chain?
|
Mayko that's true, but I do not know why. I'm not too much skilled in theory, I like practical chemistry
more. Anyway it is nice experiment https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Yep, I've been wanting to try the limonene to carvone conversion for a while, finally getting set up and want to understand more about the mechanisms
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
i want to nitrate this compound....being that the group is large and its an o,p directional i will get the p position.....my concern is if there will
be any reaction with my side chain exposed to the sulfuric and nitric acid used to place the nitro group....i.e esterification of the alcohol
function ...and it might affect the direction of the nitro placement...?........solo
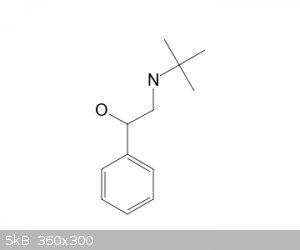
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I guess you will eliminate water forming an alkene before forming an ester. I wouldn't worry about forming an ester as it would probably not effect
direction of the nitration too much and it would be reversible.
[Edited on 18-8-2022 by Tsjerk]
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
......thanks i though of making the nitronium ion first with sulfuric acid and nitric acid then add to my compound with some cooling...solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Can BiCl3(aq) be made easily from Bi2O3? Do I need concentrated HCl or will dilute HCl do? I supposed I need an excess of the acid to prevent the
hydrolysis of the BiCl3 in solution.
Bi2O3(s) + 6HCl(aq) = 2BiCl3(aq) + 3H2O
BiCl3 is difficult/expensive to get here, but Bi2O3 is cheap and easily available.
Thanks.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I have never done said reaction but I know bismuth has a tendency to form oxychlorides. A cursory looks seems to indicate it's not as bad as zinc
where the isolation of the anhydrous chloride itself is next to impossible but seems it might be difficult to do without that showing up as a
contaminant.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quote: Originally posted by BromicAcid  | | I have never done said reaction but I know bismuth has a tendency to form oxychlorides. A cursory looks seems to indicate it's not as bad as zinc
where the isolation of the anhydrous chloride itself is next to impossible but seems it might be difficult to do without that showing up as a
contaminant. |
From what I read, the hydrolysis to form oxychlorides can be reversed/prevented by using an excess of acid.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by artemov  | Quote: Originally posted by BromicAcid  | | I have never done said reaction but I know bismuth has a tendency to form oxychlorides. A cursory looks seems to indicate it's not as bad as zinc
where the isolation of the anhydrous chloride itself is next to impossible but seems it might be difficult to do without that showing up as a
contaminant. |
From what I read, the hydrolysis to form oxychlorides can be reversed/prevented by using an excess of acid. |
Yeah, that's correct. Dissolving in 1+1 acid is okay. Maybe even 1+2 would be good.
[Edited on 27-8-2022 by Bedlasky]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
The Bismuth question is related to its iodo-complexes discussed here.
I'm also interested in these complexes, but I only have Bi metal. I tried to dissolve it in ~16% HCl, but it did not dissolve at all, neither when
boiled.
I then tried to dissolve the same piece in 53% nitric acid and it did dissolve, giving off NO as orange-brown fumes. The solution was neutralised with
ammonia solution until pH paper showed the pH was > 9. A fine, slightly off-white precipitate was formed (would do a good job as a warm white
pigment).
The precipitate was suction filtered on a piece of Whatman white band filter paper put on a coarse fritte. Approx. 10% of the precipitate ran through
during the first filtration, but virtually all was filtered when the filtrate was run through the same filter paper again. The residue was rinsed by
pouring water on it after the initial solution has run though (still under suction).
The residue was dissolved in the earlier used 16% HCl solution. Another 0.5ml 36% had to be added in order to dissolve all of the residue. Heating was
needed.
Around 10% was spilled and when the spilled drops combined with drops of water in the sink, a milky liquid immediately formed. It is evident
that in order to keep Bi in solution, an excess of strong acid is required.
[Edited on 27-8-2022 by Bezaleel]
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quote: Originally posted by Bezaleel  |
The residue was dissolved in the earlier used 16% HCl solution. Another 0.5ml 36% had to be added in order to dissolve all of the residue. Heating was
needed.
Around 10% was spilled and when the spilled drops combined with drops of water in the sink, a milky liquid immediately formed. It is evident
that in order to keep Bi in solution, an excess of strong acid is required.
[Edited on 27-8-2022 by Bezaleel] |
Thanks Bedlasky.
Bezaleel:
Yes I am trying to make the red salt cesium pentaiodobismuthate.
This is the only color missing from my rainbow salt series. Hope it really turns out red! lol
Would just adding more 16% HCl dissolve all of the ppt, or is a strong acid (>30% HCL?) absolutely necessary?
I have some <20% HCl, but not >30%. Guess I might need to make a small amount.
I bought some cesium salt (expensive!) and Bi2O3. Waiting patiently for their delivery 
Oh, I managed to make nickel chloride and lead acetate from nickel coins and lead shots using hydrogen peroxide and the respective acid (dilute
HCl/freeze distilled vinegar). Not sure if it will work with bismuth. I dun have nitric acid and dun really want to deal with NO2.
[Edited on 28-8-2022 by artemov]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by artemov  | Would just adding more 16% HCl dissolve all of the ppt, or is a strong acid (>30% HCL?) absolutely necessary?
I have some <20% HCl, but not >30%. Guess I might need to make a small amount. |
16% solution should be fine, but an excess of acid is necessary to keep Bi dissolved.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
....what can i substitute Sulfolane with? DMSO?........solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Rainwater
National Hazard
   
Posts: 799
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
What is the easiest way to use Cl2 gas given off by an electrolysis cell? Would love to turn it into HCl
"You can't do that" - challenge accepted
|
|
|
Rainwater
National Hazard
   
Posts: 799
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
What is a safe but difficult fractional distillation?
Where the 2 components are almost identical.
I've read that vinegar is difficult but has almost an 18c difference in bp and no azeotrope it doesn't seem that it should be hard. Running a liter
now.
Edit:
when looking at the vapor liquid equilibrium i see the difficulty
Should be a good test
[Edited on 3-12-2022 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
| Pages:
1
..
96
97
98
99
100
..
103 |