| Pages:
1
..
87
88
89
90
91
..
103 |
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Anyone have any references discussing the Lucas reagent and other acidic Sn2 reactions on vicinal diols?
In particular, I’m interested in the practicality of converting propylene glycol into 2-chloro-1-propanol. The secondary alcohol should be
substituted faster than the primary, but I don’t know if there are any side reactions to worry about.
Incidentally, while researching this transformation, I was unable to find any references discussing the conversion of ethylene glycol to
1,2-dichloroethane, even though this transformation is commonly suggested as a route to DCE.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Anyone have any suggestions on paints they have found chemically resistant? I am painting my fumehood soon.
Much appreciated
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
TLC Quiz
It seems that after a lot of struggle I could finaly prepare that nicotinonitrile I asked about months ago.
I ran the starting niacinamide and the crude product side by side on a tlc plate (Kieselgel on aluminum) in 6:14 denatured EtOH : Chloroform. They
each gave one spot only.
I'm curious who would guess the resulting Rf values most accurately?! 
This "challenge" is open for a few days then I'll announce the results!
Anyone for a ride?
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
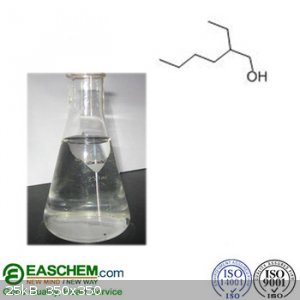
According to pubchem.gov, 2 ethylhexanol is a brown liquid, however various images of the substance show it to be clear. Which one is it? I feel like
the images are just showing a generic liquid in a flask.
Here is an example:
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
2-ethylhexanol why should it be brown? It is a simple aliphatic alcohol of which the C-chain isn't too complicated (no conjugated double or triple
bonds) so there are no chromofors.
I imagine it should look exactly like 1-octanol, maybe slightly more viscous.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Abromination  | Anyone have any suggestions on paints they have found chemically resistant? I am painting my fumehood soon.
Much appreciated |
I recently discovered "graffitiy proof" paints based on a fluorinated polymer that also takes advantage of a phenomenon I didnt look into.
It could be great for fumehoods.
The price was not so great though... Almost 200 Euros / liter !
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by Pumukli  | 2-ethylhexanol why should it be brown? It is a simple aliphatic alcohol of which the C-chain isn't too complicated (no conjugated double or triple
bonds) so there are no chromofors.
I imagine it should look exactly like 1-octanol, maybe slightly more viscous. |
Thanks, just checking, pubchem has usually been a reliable source in the past.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by bipolar  | Does acetonitrile react with sodium metal? If so, what are the products of this reaction?
I'm hoping this might be the way to make some sodium cyanide. 
[Edited on 21-4-2019 by bipolar] |
gaseous acetonitrile decomposes to HCN, a simple tube furnace (600-900C if i recall, anyway the process has 4768543983465430 references online as it
is critical for fire fighters in buildings with certain types of polymers, acetonitrile became the test standard for assessing this risk) connected to
a boiling flask of acetonitrile with the exiting gas stream cooled with a long air condenser then bubbled through two NaOH or KOH wash bottles,
indicated to clearly show when exhausted. Clever use of three way taps will allow for changeover of the solutions.
Less safe in some respects is condensing the HCN, however it does allow one greater flexibility, ie you can weigh precise amounts out to neutralise.
Be very careful, i hear HCN has deliterious health consequences to the human organism.
Always run a lengthy test cycle at temp through your setup with compressed air or something testing for leaks.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
So I have heard this a few places, some easily dismissed as technobabble (eg The X-Files) and others less so (Mythbusters iirc). This is that at high
temperatures, the dissociation of water will create hydrogen and oxygen, which will then go on to fuel and augment the fire.
Am I completely missing something??? Isn't the whole point of their dissociation at high temperature that their combination is unfavorable? Isn't any
energy released by their eventual combustion energy that the flame had previously lost? Is there some sort of potential energy transport effect I'm
neglecting?
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
@Mayko, I know this thread is dead, but I think your reasoning is right, and that theory is a crock of crap. The dissociation takes a certain amount
of heat, but as soon as the gas gets to the flametip and even cools down by a few degrees, it recombines and makes water, releasing precisely as much
energy as it took to heat it. They might as well say that pouring liquid water into a fire will heat it up, since it makes so much hot steam. The only
difference is that most laymen know about evaporative cooling, but don't know about the reversible pyrolysis of water, so poorly informed popular TV
shows can speculate about it without too many people calling BS. Now, if the steam stays well insulated at a few dozen megakelvins for years on end,
nuclear reactions will slowly heat it up. Also, if you started out with room temperature hydrogen and oxygen and mixed them, then that will obviously
heat up quite rapidly when ignited. other than those two possibilities, adding extra water into a fire will just cool it.
Dissociation does have an effect in making the peak flame temperature a bit lower, and the total moles of gas at peak temperature a bit higher, but to
say it raises the temperature or adds any energy is grossly inaccurate.
Now, I've got a question. At a hardware store, I saw some Klean-Strip "green" muriatic acid, which claimed to have 90% less fumes than normal
muriatic. The safety datasheet says it's 20% HCl. Do you think it simply gives off less fumes due to being diluted to <30%. or is there some other
additive to supress fuming?
[Edited for format, and because I just realized the last question was old)
[Edited on 6-9-2019 by C6(NO2)5CH2CH(CH3)N(NO2)2]
Put that in your pipe and smoke it!
|
|
|
magnet
Harmless

Posts: 11
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
My thermometer adapter popped up and out at the end of my last distillation because I was negligent in using a keck clip..ugh. so ordered a new one
but it's different and didnt realize the system will be open using this(I use my multimeter for thermometer). I thought of filling hole with aluminum
foil but I'm sure that would react with something sooner or later. Any ideas how to seal around wires? Sorry about volume level thru pic..damn phone
is a pitas to screen shot.
[Edited on 6-9-2019 by magnet]
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Four options.
Hot glue gun
Lots of teflon tape
Silicone sealant
Buy the adapter you want
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Heat seal (melt) the end of some 8mm o.d. borosilicate tubing and put the thermocouple in the glass tube.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
To polynitrosomeone: yes, just dilution. Why, what did you think?  No special
additives, nothing. It would cost money, anyway! Dilution is cheap - but can be explained away as the most important scientific breakthrough in the
XXI century. No special
additives, nothing. It would cost money, anyway! Dilution is cheap - but can be explained away as the most important scientific breakthrough in the
XXI century.  Like "x % less fuming"! Like "x % less fuming"! 
|
|
|
magnet
Harmless

Posts: 11
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
Thank you for the suggestions.
|
|
|
Ramium
Hazard to Others
  
Posts: 144
Registered: 3-12-2014
Location: new zealand
Member Is Offline
Mood: Licit fish
|
|
Organic reactions with chlorate?
I recently obtained a large amount of Sodium Chlorate, and have been looking for some use for it - aside from pyrotechnics. I like the idea of using
it for some kind of organic reaction, but it doesn't seem to have many uses in organic chemistry.. After a bit of searching, the only interesting use
I could find was NaClO3/HCl being used to selectively chlorinate ketones..
Any others ideas of organic syntheses which use NaClO3 a a reagent? Maybe oxidation of benzyl alcohol to benzaldehyde.. would that be possible with
chlorate?
Cheers folks,
insoluble in society
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I want to do a chiral resolution on methylbenzylamine.
However, I do not want to use a fractional crystallisation but I want to apply a selective extraction using monosodium tartrate in a two-phase system
of toluene and water.
Like this here: https://erowid.org/archive/rhodium/chemistry/amphetamine.res...
I already applied that successfully on the amine used in there, and since it was easier and quicker, I would like to use it on 1-PEA too.
Any objections?
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Question
Is lye (for soap making) NaOH or NaOH.H2O? Should I factor in the H2O when calculating stoichiometry?
According to wiki, "The commercially available "sodium hydroxide" is often this monohydrate".
[Edited on 24-10-2019 by artemov]
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Question
Is 70%-80% sulfuric acid any useful for making esters, ethers, nitric acid (from nitrates), nitrating mixture, and removing thiophenes from toluene?
What about dehydration works like drying bromine?
I have some battery acid and only able/dare to boil it down til 70-80%.
[Edited on 24-10-2019 by artemov]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by artemov  | Question
Is lye (for soap making) NaOH or NaOH.H2O? Should I factor in the H2O when calculating stoichiometry?
According to wiki, "The commercially available "sodium hydroxide" is often this monohydrate".
[Edited on 24-10-2019 by artemov] |
I did titration to check that, and now I know that some specific brand (De Parel) available on Dutch market is perfectly dry. It looks like small
globules.
[Edited on 24-10-2019 by teodor]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by artemov  | Question
Is 70%-80% sulfuric acid any useful for making esters, ethers, nitric acid (from nitrates), nitrating mixture, and removing thiophenes from toluene?
What about dehydration works like drying bromine?
I have some battery acid and only able/dare to boil it down til 70-80%.
|
Generally yes, although for nitrating mixture it might not work as well.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I found this method here for the deprotection of N-alkyl phthalimides to the primary amine: https://www.organic-chemistry.org/abstracts/literature/523.s...
Would it work with N-alkyl saccharin too?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Has anyone been able to make acetyldehyde from readily available chemicals?
|
|
|
Tellurium
Hazard to Self
 
Posts: 84
Registered: 12-7-2017
Location: Group 16, Chalcogen City
Member Is Offline
Mood: smelly
|
|
I don't know if KMnO4 is readily available for you, but I think you should be able synthesizing it by dripping EtOH in a warm/hot acidified KMnO4
solution and distilling of the Aldehyde right away. Boiling point of acetaldehyde is just 20°C, so a lot of it should escape before being oxidized to
Acetic acid.
|
|
|
Tellurium
Hazard to Self
 
Posts: 84
Registered: 12-7-2017
Location: Group 16, Chalcogen City
Member Is Offline
Mood: smelly
|
|
Well I Just cleaned a really messed up flask with H2SO4/CrO3.
The stir bar was White before(and told to be PTFE coated) and now it is completely black. Did this happen to someone before? Because I thought PTFE
was inert to this and with other stirbars this did not happen at all. Or did I maybe get ripped of with a few of my stir bars?

|
|
|
| Pages:
1
..
87
88
89
90
91
..
103 |