Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
Organic Chlorinations?
Hello! I am just getting into organic chem, and wanted to know if any of you had some good chlorination experiments to try.
|
|
|
Mercedesbenzene
Hazard to Self
 
Posts: 64
Registered: 4-9-2011
Location: BC
Member Is Offline
Mood: Sub Zero
|
|
I personally liked the radical chlorination of toluene. It chlorinates the methyl group to yield the mono, di, or tri substituted compound. All of
these are fairly useful compounds. I believe there is something on this subject under the prepublication form. Research the subject yourself to find
out some more information and probably reactions you may be more interested in.
|
|
|
(Brain)2NH
Harmless

Posts: 22
Registered: 21-7-2013
Member Is Offline
Mood: No Mood
|
|
Acyl chlorides
converting carboxylic acids to acyl chlorides, then reducing the acyl chloride to yield the mono-chlorinated methyl or even aldehydes.
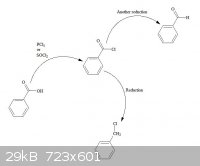
[Edited on 22-7-2013 by (Brain)2NH]
RC(O)NH2 -----------> RNH2 !
Hoffman Rearrangement
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
Thanks for the ideas, and the nice diagram!
|
|
|
bfesser
|
Thread Moved
22-7-2013 at 05:21 |
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
chlorination of acetic acid
i was looking around for this as for making nitromethane, which can seemingly be done by sodium chloroacetate and sodium nitrite
chloroacetic acid is reacted with sodium hydroxide to get sodium chloroacetate, by what i remember
although its toxic
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
Anyone have any tips on the exhaustive halogenation of a methyl ketone in basic conditions? (haloform reaction)
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Search the forum for trichloromethane (chloroform) synthesis. It is a fairly simple reaction and has been discussed here many times.
Another beginner friendly reaction would be chlorination of benzyl alcohol to benzyl chloride with excess concentrated hydrochloric acid.
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
Where can benzyl alcohol be obtained?
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Benzyl alcohol...
http://www.ebay.com/bhp/benzyl-alcohol
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
Well i mean other than buying off the internet. I only use internet as last resort.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Chlorination of dioxane is an easily made and a really well going reaction, I can just recommend it!
http://www.sciencemadness.org/talk/viewthread.php?tid=22636
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Mercedesbenzene
Hazard to Self
 
Posts: 64
Registered: 4-9-2011
Location: BC
Member Is Offline
Mood: Sub Zero
|
|
Benzyl alcohol can quite simply be made from an Sn2 reaction of benzyl chloride with some kind of hydroxide. Benzyl chloride can be made from the
chlorination of toluene
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
Ah, a chlorination necessary to perform another chlorination.... You know what that means..... CHLORINATIONCEPTION!!!!!!!
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
You have to watch the movie inception to understand what I am saying. Great mind boggler of a movie by the way!
|
|
|