cavgdad
Harmless

Posts: 22
Registered: 27-4-2004
Member Is Offline
Mood: No Mood
|
|
cleavage or hydrolosis of methoxy groups
not sure if I will state this right (not a chemist... nor do i play one on tv) so please bear with me. I would like to know if it is possible to
catalyze the removal, cleavage or hydrolosis of a mehtoxy group (R-OH?) from an allybenzene? I am interested in any method, including enzymatic or
catalytic antibodies (although prefer straight organic chem)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
It is likely to depend on exactly where on the molecule the -OH group is. What position do you have in mind? By allylbenzene, I presume you mean
2-phenylpropene, CH2=CH-CH2-C6H5. "Allyl" compounds usually refer to 2-propenyl compounds, although the bonds are different from those in
allene, CH2=C=CH2.
John W.
|
|
|
cavgdad
Harmless

Posts: 22
Registered: 27-4-2004
Member Is Offline
Mood: No Mood
|
|
cleavage or hydrolosis of methoxy groups
its 5-methoxy 3,4 methylenedioxy allylbenzene. i have done quite a bit of googling and can find no reference where a methoxy group is cleaved. its
just hard to believe. i did see a reference where the methoxy can be sustituted with a cloro group, but does that help to replace it with a hydrogen?
i suppose it has something to do with leaving group strength and protection...... anyways thats what i am looking for.
|
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
Thats what i figured you wanted to do, i really dont think thats possible without breaking or removing the 3,4 - methlyenedioxy group as well. If
their was a way to do it people would be all over parsley leaf and other things for their safrole needs instead of risking it was sassy oil.
\"I love being alive and will be the best man I possibly can. I will take love wherever I find it and offer it to everyone who will take it. I
will seek knowledge from those wiser and teach those who wish to learn from me.\" Duane Allman
|
|
|
cavgdad
Harmless

Posts: 22
Registered: 27-4-2004
Member Is Offline
Mood: No Mood
|
|

hard to believe there isn't an enzyme that has this function. but going thru the research on enzymes could take a hundred years
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That compound has the -OCH3 group alpha to the allyl group which is presumably on the "1" carbon. By 3,4-methylenedioxy, I presume you mean
that there is a 5-membered diether ring fused to the 3 and 4 carbons, [C3]-O-CH2-O-[C4].
This makes the compound an aromatic triether. But it would be difficult to do anything to the -OCH3 group, like cleaving it from the ring and
replacing it with H, without also affecting the diether ring.
The usual method of cleaving ethers is by heating with strong non-oxidizing acids like HCl, HBR, or H2SO4. In the case of HCl or HBr, the
corresponding alkyl or arl halides are formed, with elimination of water. With an excess of the ether, one of the two products would be an alcohol or
phenol. When cold contrated HI is used, the products are also an alcohol plus an alkyl or aryl iodide. In the cases of mised (asymmetric) ethers, the
latter case would result in a mixturee ofboth halides and alcohols/phenols, but the composition depends on the nature of the groups in the ether.
Unfortunately, these reactions seem to make no distinction between -OCH3 (methoxy) ethers and cyclic ethers. And because the ether groups are not the
same, in the latter two cases of reactions you would end up with a mixture of several different halides and phenols.
AND, unfortunately, the allyl group, which is alkenic, would, in the same reactions, add H and a halide across its double bond at the same time! The
halide atom would preferentially go into the most heavily carbon-substituted carbon (Markownikov Rule), which in this case would be the middle carbon
in the allyl group, but an appreciable amount (up to 10%) would also go onto the end carbon.
There may be other, more "radical", ether-cleavage methods, such as ones involving reduction of the oxygens with very powerful reducing
agents under extreme heat and pressure conditions, like H2 (this would also hydrogenate the allyl double bond), alkaline earth metals, red phosphorus,
selenium, etc.. The ones other than H2 frequently remove most or all functional groups containing electronegative atoms like oxygen.
John W.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
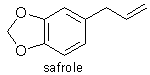
Re safrole
You mention safrole, Darkfire. It is indeed closely related to Cavgdad's compound, in fact the compound minus the -OCH3 (replaced by H). Is this
your idea? It is highly valued for perfumery and food flavoring (but poisonous in large quantities - a liver carcinogen then), and can also be used as
a starting material to make MDMA (ecstasy). The Fascist DEA is trying to have it banned in the U.S.A..
Other names for it are: 1,2-methylenedioxy-4-allylbenzene 3,4-methylenedioxyallylbenzene,
5-(2-propenyl)-1,3-benzodioxole,
4-allyl-1,2-methylenedioxybenzene,
allylchatecol methylene ether, allyldioxybenzene methylene ether,
m-allylpyrocatechin methylene ether, allylpyrocatecholmethylene ether,
1-allyl-3,4-methylenedioxybenzene.
The most common form is the trans isomer (at the double bond), although cis-isosafrole also exists.
It is the 80-85% main constituent of sassafras oil, and indeed Cavgdad's compound may be a minor constituent. Safrole also occurs in varying
amounts in the oils of cinnamon (up to 99% in some species), nutmeg (trace), juniper (about 11%), piper species (pepper, around 70% in some species
but only a trace in black pepper), magnolia (trace), etc. Gas chromatography on a packed column can be used to purify it, from these oils. To avoid
suspicion in purchasing it, you might as well grow your own sassafras, or cinnamon or piper species.
For further info about it, including properties, see:
http://www.rhodium.ws/chemistry/safrolefaq.html
http://www.rhodium.ws/chemistry/3base/safrole.plants/
http://www.scorecard.org/chemical-profiles/summary.tcl?edf_s...
http://mdma.net/safrole/
http://leda.lycaeum.org/?ID=2989
http://encyclopedia.thefreedictionary.com/Safrole
http://193.51.164.11/htdocs/monographs/vol10/safrole.html
http://193.51.164.11/htdocs/monographs/vol01/safrole.html
http://www.hort.purdue.edu/newcrop/proceedings1999/v4-479.ht...
http://www.flavornet.org/info/94-59-7.html
http://www.deadiversion.usdoj.gov/chem_prog/advisories/safro...
http://www.ussc.gov/1998GUID/safrole.htm
http://www.grokfood.com/additives/2680.htm
http://www.grokfood.com/additives/1387.htm
http://www.fasthealth.com/dictionary/s/safrole.php
http://www.inchem.org/documents/iarc/vol10/safrole.html
http://www.cognitiveliberty.org/shulgin/adsarchive/safrole.h...
http://www.hub-uk.com/cooking/tipssassafras.htm
http://www.herbs2000.com/herbs/herbs_sassafras.htm
http://www.labdepotinc.com/chemical_details~pid~S2014.aspx
http://www.shaman-australis.com.au/Website/Constituents/Safr...
http://www.essential-oil.org/shop/sassafrasvietnam.htm
http://www.dq.fct.unl.pt/qoa/bpn2002/safrole/PaginaPrincipal...
John W.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
P.S. The molecular structure of trans-safrole is attached here:
|
|
|
svm
Harmless

Posts: 31
Registered: 21-7-2004
Member Is Offline
Mood: No Mood
|
|
the most common ways to cleave a methyl ether are TMS iodide or BBr3. both are pretty nasty to deal with.
aryl methyl ethers are generally much easier to cleave than alkyl ethers. it might be doable. these methods will give the phenol though, I
don't know if that's what you want.
[Edited on 15-9-2004 by svm]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
The ether cleavage with BBr3, mentioned in the above post, seems to be still a very new and experimental method, not found in textbooks. I could find
only 30 distinct references to it (repeated in about another 100) in a Google search. These few reported uses have been mainly on methyl aryl ethers,
so it may well work here - but it would also cleave the cyclic diether part, and there may be some reaction with the allyl double bond if the
byproducts or heat decomposes the BBr3, which is likely. Another very new similar method appears to be use of AlCl4-ionic compounds.
A Google search revealed NO mentions anywhere of use of the other reagent mentioned in the above post, namely TMS iodide, for the purpose. Some
results for the cleavage mention TMS only in connection with NMR spectroscopy, or iodide referring to a different reaction.
John W.
|
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
The facist DEA isnt trying to ban it, they already did, they catch you with safrole, your sayin goodbye to the next three or four decades of your life
you anal rape and ass beatings.
Myristicin is the common name of the compound your talking about i belive, i have never come acroos a way to convert that to safrole.
\"I love being alive and will be the best man I possibly can. I will take love wherever I find it and offer it to everyone who will take it. I
will seek knowledge from those wiser and teach those who wish to learn from me.\" Duane Allman
|
|
|
cavgdad
Harmless

Posts: 22
Registered: 27-4-2004
Member Is Offline
Mood: No Mood
|
|
what about "allyl-alcohol dehydrogenase "
or maybe i should post in the biochem forum?
|
|
|
svm
Harmless

Posts: 31
Registered: 21-7-2004
Member Is Offline
Mood: No Mood
|
|
I've got five journal references for the use of TMS iodide dating from 1977 to 1981. six BBr3 references dating from 1977 to 1989 too. just
because it doesn't show up on google doesn't mean it isn't out there. (info is from this book: http://www.amazon.com/exec/obidos/tg/detail/-/0471160199/002... )
as I mentioned, I think that aryl methyl ethers are cleaved much more readily than alkyl ethers, so I would think that it would be possible to cleave
the aryl ether without cleaving the cyclic ether.
[Edited on 15-9-2004 by svm]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
| Quote: | Originally posted by Darkfire
The facist DEA isnt trying to ban it, they already did, they catch you with safrole, your sayin goodbye to the next three or four decades of your life
you anal rape and ass beatings. |
When posting outside of Whimsy, try to keep messages as factual as possible. This sort of hyperbole is not useful.
PGP Key and corresponding e-mail address
|
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
Ive talked to primo pyro and madsci, and seen a link from rhodium somewhere that saffrole is illegal in the US.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Oops, I did not read properly the passage in the Google results about safrole already being illegal, or severely restricted, in the U.S.A. (but
probably nowhere else in the world). But it is a totally useless and unenforceable law, anyway, because of the large number of natural botanical
sources from which it can be obtained in high yields, including even native juniper, magnolia and sassafras species.
John w.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I'm only going to do this once
I find it distatsteful and annoying when complex issues are simplified and then the simplified version is treated as if it is all that it exists.
Rhodium's page on "Watched and Listed Chemicals in the US" states that safrole is a DEA List I chemical. It also states that "List
I Chemicals (a.k.a. Schedule I Chemicals, a.k.a. Precursor Chemicals) are all illegal to own or buy in any quantity without a DEA or State
Permit."
This is incorrect. Some months back, I did a few hours of research to find out what the law actually said about List I chemicals (all the relevant
federal laws can be found online). My recollection is that a permit is required to import or resell such chemicals, but no permit is required to
purchase or possess such chemicals as an end user. Of course the law doesn't come out and say "no permit is required of an end user,"
but importers/exporters and resellers were the only ones I can recall who needed permits. Transactions over threshold quantities, even to end users,
must be reported, and records must be kept for all List I transactions (IIRC), but the burden is on the seller/importer/exporter, not the buyer.
Now it may well be the case that chemical suppliers want some sort of authentication before they will sell you List I chemicals, even as an end user.
But this seems to be a voluntary (if widespread) industry practice, just like many video stores won't rent R-rated movies to people under 17. In
both cases there seems to be a very popular misconception that this widespread practice is actually a LAW. It isn't. Individual states may
criminalize possession of List I chemicals without a permit (I haven't tried to do state by state research), but I am reasonably certain that
federal law does not. Proof by example: benzaldehyde ("artificial oil of bitter almonds" is a List I chemical and a widely used flavor and fragrance material. It is available for purchase from a variety of
Internet businesses and in person from many well-stocked cooking or craft stores. A couple of months ago I bought a small quantity of it at a local
candy and cake shop. It was no more difficult than buying vanilla or lemon flavoring. is a List I chemical and a widely used flavor and fragrance material. It is available for purchase from a variety of
Internet businesses and in person from many well-stocked cooking or craft stores. A couple of months ago I bought a small quantity of it at a local
candy and cake shop. It was no more difficult than buying vanilla or lemon flavoring.
I raised the issue of the misleading statements about List I chemicals on the Hive's Law and Order forum, but nobody seemed willing to take the
time to verify my research or acknowledge that there was a flaw on the Watched and Listed Chemicals page. Maybe the goal is more to scare would-be
drug chemists away from buying certain chemicals than it is to accurately report the law in the US. "Better safe than sorry, even if we have to
fib" ELL OH ELL.
Or maybe the page is written the way it is because every US citizen the author ever knew, who was caught with List I chemicals, did face some sort of
legal penalty. The possession of listed chemicals could be a significant piece of evidence in prosecuting someone for the manufacture of controlled
substances, after all. Whatever the reasoning, the page is wrong as currently worded. "Possession with intent to manufacture [controlled
substances]" is different from plain possession.
In some instances, prosecutors may build a case for intent to manufacture with flimsy evidence (including mere possession of certain chemicals with no
evidence of controlled end products ever being created), but plain-and-simple possession of safrole or any other List I chemical is not a crime.
Further, even for people convicted of manufacturing controlled substances, 30-40 years is at the upper end of sentences imposed. It is very wrong to
imply that someone will be imprisoned for 40 years because they have a liter of safrole stored between the rhodamine 6g and the tetrabutylammonium
bromide.
[Edited on 9-15-2004 by Polverone]
PGP Key and corresponding e-mail address
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
| Quote: | | (cut) Myristicin is the common name of the compound your talking about i believe, i have never come across a way to convert that to safrole.
|
You are right; Cavgdad's compound IS myristicin, 3-methoxy-4,5-methylenedioxy-allylbenzene, which is the principal active constitutent of nutmeg
oil (along with similar compounds like eugenol and elemicin and some safrole, and lipids like that of myristic acid, the straight-chain C-14
carboxylic acid). At first I thought it might be a structural isomer, but on turning over the molecule like Cavgdad, it is the same thing, 5-methoxy
3,4-methylenedioxy-allylbenzene. It is also found in some other plants in small amounts, e.g. cloves, and with anisole and selinene in dill and
parsley and fennel etc..
It is hallucinogenic in amounts much larger than those used as spice in foods. Because it can be steam-extracted from ground nutmeg (of which it
comprises 4%) cheaply available as a culinary spice in all supermarkets, and can be purified by vacuum distillation (or chromatography), there is
little chance of the DEA being able to get it banned or restricted.
For further information:
http://www.omikron-online.de/cyberchem/aroinfo/myristic.htm
http://leda.lycaeum.org/?ID=2990
http://www.rhodium.ws/chemistry/nutmeg.myristicin.html
http://leda.lycaeum.org/?ID=13452 (extraction)
http://ntp-server.niehs.nih.gov/htdocs/Chem_Background/EXECS...
http://www.flavornet.org/info/607-91-0.html
http://www.shaman-australis.com.au/Website/Constituents/Myri...
http://www.erowid.org/chemicals/myristicin/
http://www.myristicin.com/
http://myristicin.wikiverse.org/
http://www.catbull.com/alamut/Lexikon/Mittel/Myristicin.htm
http://www.fslemi.uni-bonn.de/gewuerze/html/stralshtml/myris...
http://www.medicinescomplete.com/mc/herbals/current/noframes...
It can be synthesized from eugenol, the principal constituent of oil of cloves (eugenia), which the DEA would find impossible to ban or restrict:
http://www.rhodium.ws/chemistry/eugenol2myristicin.html
It may be difficult or impossible to convert myristicin to safrole by removing the -OCH3 group without removing the cyclic diether group. But, would
it be possible to make MDMA (3,4- methylenedioxy-methamphetamine, chemically similar to the stimulant methamphetamine and the hallucinogen mescaline)
from myristicin or eugenol, or some other naturally common benzene ether, preferably already having the cyclic methylene diether group, without having
to convert to safrole first?
John W.
[Edited on 16-9-2004 by JohnWW]
|
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
MDMA is only similar to meth by its structure not by its action on the body. Theres basicly 2 ways to MDMA, safrol or 3,4-MD-benzaldahyde. Other
things like eugenol other exotic things but its much less practical.
\"I love being alive and will be the best man I possibly can. I will take love wherever I find it and offer it to everyone who will take it. I
will seek knowledge from those wiser and teach those who wish to learn from me.\" Duane Allman
|
|
|