Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
Conversion of Aldehydes to Nitriles
Hey everyone,
I came across this article: "A Practical and Cost-Efficient, One-Pot Conversion of Aldehydes into Nitriles Mediated by 'Activated DMSO' ", Synlett,
2011, 2223-2227
Now, I don't typically trust these sorts of papers, but I was wondering if this is theoretically feasible. I don't have access to the whole paper, but
I want to propose a potential reaction scheme (based on this article). The scheme is just an example, but I thought it important to add that I'm more
interested in 3-phenylpropanenitrile and the corresponding ring-substituted relatives.

In this scheme, (a) represents the initial oxime formation phase. I was thinking of using hydroxylamine hydrochloride and a carbonate salt (probably
Na2CO3). I just need a base strong enough to deprotonate hydroxylamine hydrochloride. I have performed this reaction in methanol, ethanol and
isopropanol previously, and I know these conditions will give the nitrile compound.
For (b), I didn't plan on using any other reagents. This paper makes the assumption that the oxime will dehydrate on its own if given enough heat.
They reported 90oC, but my plan is to hit above 100oC (~110oC). I know that Vogel has a prep using acetic anhydride to promote conversion of the oxime
to the nitrile. My understanding is that the acetyl ester leaves as acetic acid, which is easier to do than to try to strip away the hydroxyl group
from the oxime nitrogen without any assistance. As a side note, the authors make no mention of added reagents to promote formation of nitrile. I am
not sure what they mean by 'activated DMSO.'
The workup would consist of adding a large amount of ice-water to the mixture to precipitate the intended product out. If precipitation does not
occur, I plan to extract with DCM and then wash the organic layer with brine to remove excess DMSO.
EDIT: The link was broken.
[Edited on 6-9-2013 by Rich_Insane]
[Edited on 6-9-2013 by Rich_Insane]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Just be careful, as hydroxylamine hydrochloride and hydroxylamine tend to explode when heated, so ensure that you don't have excess material left, and
I would heat it behind a blast shield as a precaution. A large hydroxylamine plant blew up last year, so even the "experts" have problems with it.
Making the oxime should be easy, the dehydration does not look simple. I would expect that you would need some harsher reagents, normally, but it may
well work due to the heat driving off the water to shift the equilibrium.
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
I don't plan on working with anything above 500 mmol of substrate, and I plan to not use excess hydroxylamine. Usually, I isolate the oxime
separately, but here, it seems that dehydration works fine without isolation.
I found: (Attached)
This paper is from a Chinese Journal, but I am somewhat convinced of its legitimacy because I know that acetic anhydride is often used to dehydrate
oximes. Would other, more accessible acid anhydrides work to aid dehydration of oximes?
I was thinking along the lines of phthalic anhydride, maleic anhydride and succinic anhydride. I could probably get all of these from a supplier on
Ebay or something. I still plan to take the one-pot approach in DMSO. Since DMSO is rather high-boiling, I will be able to approach >100oC
temperatures. I do not plan to go up to 160-170oC as this Chinese paper suggests. That seems like overkill to me.
Another option I'd like to explore is the possibility of using trichlorotriazine. I found a method from "J. Org. Chem.
2002,67, 6272-6274" that uses trichlorotriazine in DMF at room temperature to facilitate conversion of ketoximes into amides and conversion of
aldoximes into nitriles. The authors report nearly quantitative yields for the nitriles. What I am worried about is the possibility that
trichlorotriazine might be impossible for the OTC chemist to obtain. I am familiar with a TCCA/aq NH3 mechanism of conversion from aldehydes to
nitriles, but I have spoken to a friend who has reported dubious results using this route. With this route, I'd have to use DMF instead of DMSO, as
DMF plays an active role in the reaction. How OTC is the trichlorotriazine? I've never heard of it being used commercially, but I have noticed that it
is rather cheap when purchased from major chemical suppliers.
EDIT: Link does not work, see the attachment.
[Edited on 6-9-2013 by Rich_Insane]
Attachment: paper_8003_1269165638.pdf (118kB)
This file has been downloaded 1087 times
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by Rich_Insane  | | I am familiar with a TCCA/aq NH3 mechanism of conversion from aldehydes to nitriles, but I have spoken to a friend who has reported dubious results
using this route. |
Since its probably in situ formed NCl3 you may have a look into a similiar method using in situ formed NI3 for nitrile synthesis?
iodine ammonia h2o2 aldehyde to amide.PDF (50kB)
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
I have worked a lot with hydroxylamine (in pure form) and with it salts, it is a pretty good reagent, easy to work with.
Pure hydroxylamine is made by adding triethylamine to the suspension of hydroxylamine.HCl in dichloromethane at -20°C. A layer will separate at the
top of the dichlorometane, this is pure hydroxilamine. Do not heat it or scratch the wall of the glass. The best is to dissolve it in something and
separate it from the organic layer.
The nitrile formation from aldehydes with hydroxylamine goes pretty easily. Just dissolve the aldehyde in a good solvent (DMSO, ACN, NMP, toluene,
xylene, ect), add the hydrolyamine.HCl and a weak base (e.g.: sodium-acetate or carbonate, maybe TEA), stir it for a long time (in some cases the
oxime forms really slow, check it on TLC!) and when the oxime is formed, somehow remove the water with some catalytic amount of acid, pTsOH often
works well. In DMSO, NMP just boil up the solution, or if you work in xylene or toluene, then use a Dean-Stark trap to catch the water.
Sometimes to remove the water some phthalic anhydride or acetic anhydride is needed to be added, this often good if you work in ACN.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Why nitrile? I understand interest in helional's oxime, or nitrile, but not both. Covering all the bases? The nitrile can be made in other ways.
[Edited on 8-9-2013 by S.C. Wack]
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
The aldoxime can be converted (here the oxime will be an intermediate) to the nitrile by dehydration.
| Quote: |
The nitrile formation from aldehydes with hydroxylamine goes pretty easily. Just dissolve the aldehyde in a good solvent (DMSO, ACN, NMP, toluene,
xylene, ect), add the hydrolyamine.HCl and a weak base (e.g.: sodium-acetate or carbonate, maybe TEA), stir it for a long time (in some cases the
oxime forms really slow, check it on TLC!) and when the oxime is formed, somehow remove the water with some catalytic amount of acid, pTsOH often
works well. In DMSO, NMP just boil up the solution, or if you work in xylene or toluene, then use a Dean-Stark trap to catch the water.
|
I've performed the oxime formation with sodium carbonate (and sodium acetate) and hydroxylamine hydrochloride in alcohols (iPrOH, EtOH, MeOH), but
never in DMSO. In alcohols, usually a viscous yolk-colored oil forms and settles on the bottom. Addition of water causes more of the goo to
precipitate out, and some of it begins to float. After about 24 hours, the goo solidifies, and I filter to isolate the oxime. I want to use DMSO
because I ordered it, and it has arrived. You say p-TsOH will work? Will other acids work as well (i.e H2SO4)? I can get TsOH, but not very cheaply. I
can get acetic anhydride as well, but it is somewhat expensive and also a listed chemical. I don't know if I can get phthalic anhydride. How much
anhydride will I need to remove water?
The ultimate goal of nitrile synthesis is to convert the aldehyde to an amide. I wonder if I can perform this reaction by isolating the oxime (maybe
purifying it too) and then performing a one-pot reaction that proceeds via formation of the nitrile. In the past I have used metal-catalyst based
means of doing so (especially copper-II catalysts), but I wonder if I could do something like this:
Oxime dissolved in DMSO --> Nitrile --> Amide via addition of aq. sodium percarbonate (giving Na2CO3 and H2O2 upon dissolution). The sodium
carbonate should in theory neutralize the acidic byproducts left over from the previous step. The amide is rather insoluble in water (relatively
insoluble), so I could add enough water to dilute the DMSO and then extract with ethyl acetate or DCM.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
But there are already one-pot methods for helional to the amide that gives MDA with the Hofmann? Isn't the Beckmann of the oxime the most discussed
route to the amide?
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
I'm familiar with the 2-Methyl-3-(3,4-methylenedioxyphenyl)propanal route to the amide. In fact, I have already explored the modified Beckmann of the
oxime using metal catalysts (the one-pot route doesn't typically work very well for that by the way). My purpose is to extend methods to other
aldehydes as well as explore new routes. The modified Beckmann requires metal catalysts and high temperatures -- my experience has been negative
working with this route. Typically, at least with copper catalysts (copper acetate and zinc acetate appeared to have been the most successful), I end
up with a "goo" that requires significant time to extract and purify the amide. I also do not have a normal laboratory hotplate, and am using a
cooking stove. This makes regulating high temperatures very difficult.
My goal is more to discover one-pot or at least semi-one-pot routes that work without utilizing high temperatures. I will attempt the modified
Beckmann using DMSO (unlike xylene or toluene, DMSO will actually solvate the metal catalyst, allowing me to potentially use a water bath constant at
100oC instead of an uncontrollable sand bath) however. At this time, I'm exploring the nitrile route because I can develop a one-pot method.
Goes something like this:
The oxime is synthesized in methanol and recrystallized if necessary. This oxime is dissolved in a small volume of DMSO and the DMSO is heated to
120oC for 2 hours, then left at 100oC for 6 or so with addition of an acid (p-TsOH as kristofvagyok has suggested). A small amount of phthalic acid
anhydride might be added as well, or perhaps only phthalic anhydride will be used.
Alternatively, the oxime can be formed in situ following the protocol in the paper I have cited.
After this, a solution of sodium percarbonate in water or DMSO is dripped into the nitrile-DMSO solution. The vessel is held at 50-60oC for a couple
of hours. This refers to a paper which I referenced in another post. If the amide is insoluble in DMSO, it should crash out upon formation.
Otherwise, ice water or briney ice water will be added to facilitate precipitation.
In order for me to develop this protocol more effectively, I will need access to this paper:
"A Practical and Cost-Efficient, One-Pot Conversion of Aldehydes into Nitriles Mediated by 'Activated DMSO' ", Synlett, 2011, 2223-2227
Neither my workplace nor my school has access to this journal, for some odd reason. Can anyone help out?
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
EDIT (Sorry for the double post guys, the site won't let me edit my post)
I'm looking for a little guidance. I've just found that trichlorotriazine is sold as cyanuric chloride. I've discovered two articles that describe
utilizing trichloro-s-triazine to catalyze the synthesis of nitriles/amides from oximes. Because I am working with aldoximes, not ketoximes, the
product of the Beckmann rearrangement will be a nitrile, am I right? If this is true then "J. AM. CHEM. SOC. 2005, 127, 11240-11241" would apply.
In this paper, the authors describe the use of catalytic cyanuric chloride and zinc(II)chloride as a co-catalyst. The reaction is carried out in
acetonitrile, but they say that other polar, nucleophilic nucleophilic solvents work.
Now, I'm looking at the mechanism of this reaction, and it's starting to occur to me that the nitrile isn't even an intermediate in the reaction.
Essentially, there is an activation of the hydroxyl group on the oxime and a complex between TCT and two oxime molecules forms. The amide is then
ejected as a product and the cycle continues. My assumption is that the ZnCl2 serves as a catalyst for the nucleophilic aromatic substitution. Could
this mean that the reaction gives direct access to primary amides? Would DMSO work as a solvent here instead of CH3CN, or would TCT react with the
DMSO?
I've also found another article that's slightly older. This article specifically pertains to dehydration of aldoximes via cyanuric chloride ("Chem.
Comm. 1972, pp 1226-1227"). Here, the procedure is much simpler. The TCT is dissolved with the oxime in CH2Cl2 and pyridine is added. Reaction
proceeds at room temperature. I'm not sure what the pyridine is for. For starters, pyridine is somewhat difficult to procure for me, but I'm not sure
what it does here. This reaction is also quite good because it a) proceeds at r.t. and b) takes 1-2 hours to run to completion. Again, I want to run
this reaction in DMSO, though I could use CH2Cl2 if it is absolutely necessary (it's a lot easier to remove CH2Cl2 than it is to remove DMSO). This
article contradicts my previous speculation of direct access to the amide, as here the presence of TCT seems to drive the reaction towards the nitrile
and not the amide. I'm not sure if adding ZnCl2 will make any difference.
Any input from you guys? I'm not very good at organic chemistry and help would very much be appreciated. Sorry if I'm missing something trivial in my
ramblings 
[Edited on 10-9-2013 by Rich_Insane]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Just a little note here, oximes of aldehydes normally exist in the form of the trimer, but under heating/melting this decomposes into the monomer,
which is actually the nitroso tautomer, and this typically exists in the form of a pseudonitrosole dimer. The monomer can readily polymerize back to
the trimer. So the structure of an aldoxime is probably not actually truly an aldoxime (-CH=NOH).
However, it is probable that any dehydration to a nitrile does indeed proceed through the (true) aldoxime tautomer.
So in a heated solution of 3-phenylpropanaldoxime, there likely exist a mix of a monomer, dimer, and trimer, all with very different structures, and
none of which are technically 3-phenylpropanaldoxime. 
But perhaps being solvated in DMSO makes the aldoxime tautomer more favorable and facilitates dehydration.
[Edited on 10-9-2013 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Now, if you wanted to convert a nitrile into an aldehyde...
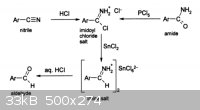
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
I have made a substitued phthalaldehyde on this way, it's a really good method, the only problem, that a lot dry hydrogen chloride is needed. A good
recipe is found here: http://www.orgsyn.org/orgsyn/prep.asp?prep=cv3p0626
HCl gas cylinder recommended.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
lexeym
Harmless

Posts: 1
Registered: 11-1-2013
Location: Moscow
Member Is Offline
Mood: No Mood
|
|
aldehyde->oxime:
dissolve your aldehyde in pyridine, heat this solution to reflux, add 1.5 eq of hydroxylamine hydrochloride, then reflux for 5 minutes. If aldehyde is
insoluble in pyridine just filter it and wash with water. If it is soluble, neutralize pyridine with diluted HCl and extract compound into diethyl
ether. TLC is recommended to check product formation. I made also oximation of ketones, it was enough 3 days at RT in pyridine for them.
oxime->nitrile:
reflux your aldoxime in 15-20 eq of acetic anhydride, evaporate remained Ac2O and AcOH. Recrystallize or distill your product. Also another
water-trapping reagents such as DCC (1 eq, EtOAc, RT, 1 day) can be used for this dehydration
|
|
|
|