| Pages:
1
..
28
29
30
31
32
..
76 |
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Hopefully some Tetracyanocuprate(II). It's said to be purple if you find anything mention about it in Literature. Usually you'll get Cu(I)Cyanide and
then Tetracyanocuprate(I) which should give you a green to orange solution depending on the conc. of KCN. But obviousely although not very stable over
time you can get Cu(II)Cyanide, too. Which I think has a really beautiful color. I even got a darker version but I couldn't get it on cam and it was
more intense than on the picture. I guess my camera doesn't really pick up colors that well.
So I found 2 short sentences in Literature. The Cu(II)Cyanide is yellow and Cu(I) is white. And the complex is purple. So this should be the complex.
I did some yellow-green precipitate, too that later turned puprle when I stirred it. So this could be Cu(II)Cyanide but I'd say that Cu(I)Cyanide does
appear a quite similar color. So not sure about these two but well the Cuprate(II) seems to exist with it's purple color.

[Edited on 23-3-2015 by fluorescence]
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Oh I never won anything with chemistry except some elementary school competitions. I selected my friends by looking at their hands during these
competitions. The more nitric acid bite marks on their hands the more likely they knew the score, today its kinda the opposite
Anyway after the termite burned I dropped it onto some water, it bounced and gave off a moderate "Puff" due to elemental sodium, it is good to have
kids around to have them grind the Mg powder off the 2kg block though.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
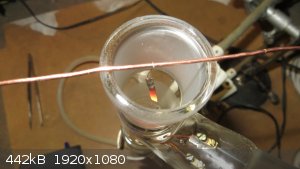
Oxidation of ethanol vapors with Pt foil 
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Two chemistry books I luckily acquired!
One is from 1913 (the one on the left) and the other about organic chemistry is from 1908!
So cool, and they were so cheap!

Pieces of history, pre-world war, so cool to own, i was so happy the day i found them (dusted, in an antiquarian bookshop) 
[Edited on 25-3-2015 by karlos³]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
He was just saying that
you probably would have won the Rador Labs Chemical Conflagrations challenge had you entered that picture. I would have to agree. Since you seem to have a knack for photographing
chemistry, you may want to keep an eye out for our future challenges, which may involve similar things.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by zts16  | | He was just saying that
you probably would have won the Rador Labs Chemical Conflagrations challenge had you entered that picture. I would have to agree. Since you seem to have a knack for photographing
chemistry, you may want to keep an eye out for our future challenges, which may involve similar things. |
I just passed the local portion of the Chemistry olympiad, and will be moving onto the nationals. If I do well in that, I get a trip to Colorado....
Nice work Jimmy!
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
that looks like an induction coil there, nice. If you will, what is the rest of the setu? Does the oxidized gas get channeled to another reaction?
Also, I read that Mg dust embedded in the skin (lungs by assumption) can cause local malignant tumor growth. I recommend a facemask/respirator for any
assistants who do not know/are uncomfortable with the nature of the dust in grinding. Goggles too maybe, like swimming goggles perhaps?
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
So these aren't home-lab photos but I'm pretty stoked about them.
A couple shots of a few molecules I've been working with for the past two or so months.
First picture is compound thats red on the column and purple in solution(base compound). Please don't judge the column pack job it was a quick
separation. Chloroform to remove a fast runner and methonal to wipe the solid phase (zwitterionic isomer is sticky) which is why there is half
semi-white(stationary phase) and half red (target).
The second picture is a conjugate that is purple in solution(multistep synthesis), and the other is red in solution(prosthetic compound for tests).
  
[Edited on 5-4-2015 by smaerd]
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
Here are some beautiful pictures of Nitrocellulose flames:
https://www.dropbox.com/s/48fcoxrxrxgtvj2/1.PNG?dl=0
https://www.dropbox.com/s/w5yq8luw1m7ji2g/2.PNG?dl=0
https://www.dropbox.com/s/njb9x4vld37mglr/4.PNG?dl=0
I have a few more (and a video with slow motion) here:
https://hobbychemistry.wordpress.com/2015/04/03/burning-nitr...
EDIT: Can't post pictures, not exactly sure why...
[Edited on 7-4-2015 by HgDinis25]
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Very nice Picts. HgDinis25.
The Video with the slow motion effect was also quite fun to watch.
Nice job.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
Quote: Originally posted by Zombie  | Very nice Picts. HgDinis25.
The Video with the slow motion effect was also quite fun to watch.
Nice job. |
Thanks! I'll make sure to place more pics here, every now and then 
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
https://www.dropbox.com/s/gs1qhfkryx9ro9l/SAM_0867.JPG?dl=0
https://www.dropbox.com/s/1pagy6vy6q0iy6b/SAM_0869.JPG?dl=0
https://www.dropbox.com/s/uzkkhfhyskyo5ug/SAM_0872.JPG?dl=0
Can anyone guess what these are?
Hint:
https://hobbychemistry.wordpress.com/2015/04/09/synthesis-of...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Nice crystals, and nice write-up, but please don't capitalize the names of compounds or elements. They aren't people.
Also, you mention "tin(II) dioxide" in your write-up. That should be tin dioxide, or tin(IV) oxide.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
Quote: Originally posted by DraconicAcid  |
Nice crystals, and nice write-up, but please don't capitalize the names of compounds or elements. They aren't people.
Also, you mention "tin(II) dioxide" in your write-up. That should be tin dioxide, or tin(IV) oxide. |
Hehe the capitalization is a trend of mine and my own language. They aren't people but they are chemicals.
The Tin Dioxide part was bad though  Thanks for the heads up! Thanks for the heads up!
Glad you liked the crystals. By the way, does anyone know why I can't put images in this post? I place these links as images and they don't show up.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by HgDinis25  | Quote: Originally posted by DraconicAcid  |
Nice crystals, and nice write-up, but please don't capitalize the names of compounds or elements. They aren't people.
Also, you mention "tin(II) dioxide" in your write-up. That should be tin dioxide, or tin(IV) oxide. |
Hehe the capitalization is a trend of mine and my own language. They aren't people but they are chemicals.
The Tin Dioxide part was bad though  Thanks for the heads up! Thanks for the heads up!
Glad you liked the crystals. By the way, does anyone know why I can't put images in this post? I place these links as images and they don't show up.
|
nice job, the crystals look great! Be sure to lacquer them if you want to preserve them.
The capitalization is something I do all the time. It's a hard habit to break, I understand. Somewhere during when I was homeschooled, I forgot that
each word wasn't capitalized, and the difference between its and it's... 
|
|
|
Argentum
Harmless

Posts: 36
Registered: 18-9-2014
Location: El culo del mundo
Member Is Offline
Mood: UV light
|
|
Some nice Mn(NO3)2 crystals
Nice but contaminated, though.

|
|
|
blargish
Hazard to Others
  
Posts: 166
Registered: 25-9-2013
Location: Canada
Member Is Offline
Mood: Mode Push
|
|
Not the best quality pic in the world, but a cool effect seen when dark-brown crystals of potassium tetraperoxochromate(V) are put into hydrochloric
acid, with the formation of a blue chromium oxide diperoxide complex: CrO(O2)2.

BLaRgISH
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
I love this thread!
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
SupaVillain
Hazard to Others
  
Posts: 171
Registered: 28-10-2014
Member Is Offline
Mood: No Mood
|
|
The Sodium 2,4-Dinitrophenolate I used to sell on Ebay... still for sale just not on Ebay

Oh.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Strontium nitrate and shellac mixture; not the prettiest, but still very nice to look at:
Won't let me rotate picture for some reason
[Edited on 17-4-2015 by greenlight]

[Edited on 17-4-2015 by greenlight]

|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
nice, care to edit in a description of the pic? lovely color
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by fluorescence  | Hopefully some Tetracyanocuprate(II). It's said to be purple if you find anything mention about it in Literature. Usually you'll get Cu(I)Cyanide and
then Tetracyanocuprate(I) which should give you a green to orange solution depending on the conc. of KCN. But obviousely although not very stable over
time you can get Cu(II)Cyanide, too. Which I think has a really beautiful color. I even got a darker version but I couldn't get it on cam and it was
more intense than on the picture. I guess my camera doesn't really pick up colors that well.
So I found 2 short sentences in Literature. The Cu(II)Cyanide is yellow and Cu(I) is white. And the complex is purple. So this should be the complex.
I did some yellow-green precipitate, too that later turned puprle when I stirred it. So this could be Cu(II)Cyanide but I'd say that Cu(I)Cyanide does
appear a quite similar color. So not sure about these two but well the Cuprate(II) seems to exist with it's purple color.
[Edited on 23-3-2015 by fluorescence] |
Interesting to see that this is a purple compound, as cyanuric acid and copper(II) also combine to form a purple compound. So, the purple colour may
well arise from the N-Cu bond, when N is also bonded to C.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
1 gram flask cracker's mid-pop made from potassium permanganate, sulphur, and aluminum flash:
[Edited on 17-4-2015 by greenlight]

[Edited on 17-4-2015 by greenlight]
[Edited on 17-4-2015 by greenlight]

|
|
|
neptunium
National Hazard
   
Posts: 985
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
perhaps a high speed footage would be interesting as well .. but must be over 16000fps
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Yes, would be good, unfortunately don't have a good enough camera for this
|
|
|
| Pages:
1
..
28
29
30
31
32
..
76 |