| Pages:
1
..
62
63
64
65
66
..
76 |
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Lovely, Pok. Thanks for these.
(subscribed.)
[Edited on 4-3-2019 by j_sum1]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by mayko  | Hot off the press, a never-before-published photograph of plutonium tetrafluoride! This was obtained by nuclear anthropologist Martin Pfeiffer via
FOIA. It's a little disappointing (it is known as 'pink cake'; this is apparently misleading.) but upthread there are some very nice pictures of
exotic things like plutonium trichloride
If you're on twitter and interested in nuclear history/technology/sociology, definitely check him out!
|
Martin has a new batch of pictures, probably the only of this plutonium compound in the public record.

Announcement:
https://twitter.com/NuclearAnthro/status/1107414103499276288
More here, under My FOIA Responsive Documents/PuF4 Plutonium Tetrafluoride Pictures/
https://osf.io/46sfd/
Some chemical background:
https://www.dropbox.com/s/829rbeoruo3a78w/Preparation%20plut...
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
100%?! did they really manage to remove every atom of impurites?!
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
To three significant figures, why not?
Now if he had said, 100.00000000000%, I would be suspicious.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
No, they just rounded off to the nearest whole percentage.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
yesterday (a few hours ago) was my birthday, my best friend gave me this as a gift, Autunite sample, i've been looking for one one ebay for years,
this made me so happy
(crappy photos taken with phone, i'll take better photos with a real camera in the morning, just came home after the party hahahhaa)
  
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Wow, that's a beautiful sample, I'd love to len one, too  . .
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
It just means it is between 100.5% and 99.5%, obviously it can't be more than 100, so it is 99.5 or higher. Just like the other one which is between
93.5 and 94.5%.
|
|
|
Chem Science
Hazard to Others
  
Posts: 122
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
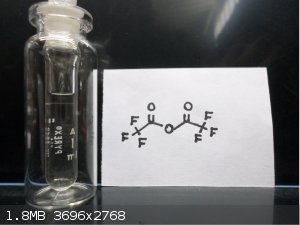
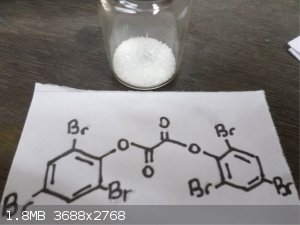
Here's some Trifluoroacetic anhydride  and bis(2,4,6 Tribromophenol) Oxalate and bis(2,4,6 Tribromophenol) Oxalate

|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
KMnO4 solution 
[Edited on 26-3-2019 by greenlight]

[Edited on 26-3-2019 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
HgCl2 after a broken mercury thermometer dissolved in an excess of HCl left for a month.

|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Yikes greenlight, you really should neutralize stuff like that instead of pouring straight down the drain! I know it probably wasn't
much, since KMnO4's color is so intense, but in this case it's easy: just add sugar!
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by MrHomeScientist  | | Yikes greenlight, you really should neutralize stuff like that instead of pouring straight down the drain! I know it probably wasn't
much, since KMnO4's color is so intense, but in this case it's easy: just add sugar! |
It would decompose in minutes when mixing with dirty drain waters.
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
some iodine in an evauated and sealed test tube (while cold it is transparent with solid iodine on the walls)

---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by MrHomeScientist  | | Yikes greenlight, you really should neutralize stuff like that instead of pouring straight down the drain! I know it probably wasn't
much, since KMnO4's color is so intense, but in this case it's easy: just add sugar! |
Yeah I dont usually just pour stuff down the drain but it was a 0.02M solution and the colour is indeed intense even with very small amounts of
solute.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
I found a picture from years ago from my first reaction ever, I must have had no clue what I was doing. I had seen a video showing copper sulfates
reaction with bases and figured since baking soda was a base, it would react with the copper sulfate. I must have been about 12. Now that I think of
it, the beautiful colors of copper compounds are really what got me interested in chemistry all of those years ago.

[Edited on 4-7-19 by Abromination]
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Wizzard1
Harmless

Posts: 10
Registered: 13-2-2019
Member Is Offline
|
|
By-product of Neodymium Sulfate purification. Enjoy!! It was about 3" across.

|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
So is it FeSO4 or Pr2(SO4)3?
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Sometimes the simple things are most satisfying.

It is nice to be back in the lab.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Beautiful, Wizzard1! I'm quite familiar with those from all my magnet work a while back. Mine never stayed nice for long.
It's FeSO4, fusso. You get tons of it from magnet processing. Pr is in such small quantities you'd need a hell of a lot of magnets to make
that much, not to mention separating and purifying it from the Nd.
|
|
|
bipolar
Harmless

Posts: 24
Registered: 24-3-2019
Member Is Offline
|
|
Polycrystal of 5-MeO-DMT freebase :3

This beauty weighs about 380 mg 
Made it from melatonin (1. hydrolysis; 2. reductive amination with formaldehyde / NaBH4).
[Edited on 21-4-2019 by bipolar]
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by bipolar  | Polycrystal of 5-MeO-DMT freebase :3
This beauty weighs about 380 mg 
Made it from melatonin (1. hydrolysis; 2. reductive amination with formaldehyde / NaBH4).
[Edited on 21-4-2019 by bipolar] |
Amazing crystal and job. Can you please provide full procedure? I would love to get back to organic chemistry as I have access to NaBH4 now. 
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Some crystals of salicylic acid, grown from toluene/hexane. i was *trying* to make butyl salicylate, but I got these instead.

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Are you sure that's salicylic acid? When I recrystallised some of mine, I got needle-like crystals. Admittedly, I don't recall which solvent I used.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by DavidJR  | | Are you sure that's salicylic acid? When I recrystallised some of mine, I got needle-like crystals. Admittedly, I don't recall which solvent I used.
|
The melting point checked out (although I didn't do a mixed one). From water, I usually see needles.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
..
62
63
64
65
66
..
76 |