| Pages:
1
..
73
74
75
76 |
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Another attempt at getting nice crystals of Ni(bipy)3SO4. The dark blue(?) crystals are presumable nickel with 2 bipy and either water or sulphate as
the other ligands.

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
First go at Picric Acid.


"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Beautiful canary yellow colour indeed, love it. 
Has a nasty habit of staining everything though haha.
Had a wet batch out drying on minimal paper towels when I was a teenager in my closet and it soaked through. The carpet is still a pretty yellow all
these years later.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|

Tin beads, created during an attempt to melt some tin.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
somehow i managed to not get yellow stains on my skin, but my god, glass with no discernible residue on it shed yellow like mad when being (re)washed.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Raid
Hazard to Everyone
  
Posts: 201
Registered: 14-11-2022
Location: N/A
Member Is Offline
Mood: School
|
|
here are some photos from under the microscope
 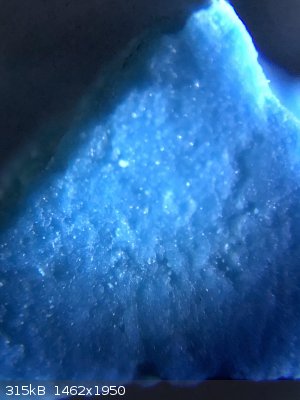 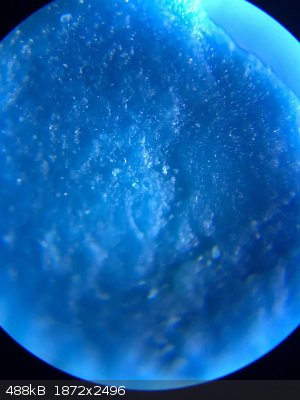  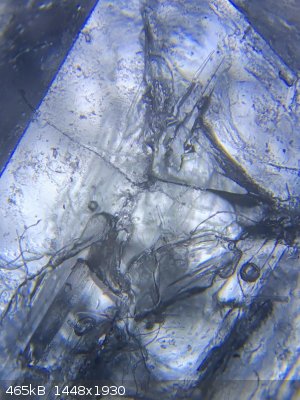 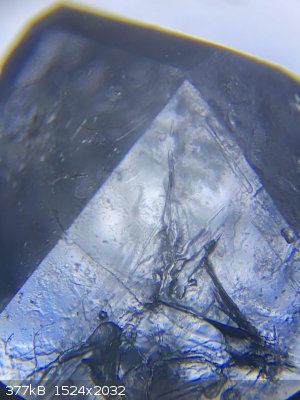 
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by arkoma  |
somehow i managed to not get yellow stains on my skin, but my god, glass with no discernible residue on it shed yellow like mad when being (re)washed.
|
You dont necessarily see the stains on your skin with normal light.
Try a black light for some fun 
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Nice photos!
How did you prepare your slides?
Did you use a stereo microcope?
What magnification(s) did you us?
Sorry for all the questions, but I have been thinking about similar observations for some time.
Charlie
|
|
|
Raid
Hazard to Everyone
  
Posts: 201
Registered: 14-11-2022
Location: N/A
Member Is Offline
Mood: School
|
|
I just kinda plopped the crystals on my microscope slide and held my camera up to the observation area and took a picture.
I used between 40x zoom and 100x
[Edited on 16-2-2023 by Raid]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
It's not difficult to clobber up a "stand-off" from your objective lens with a bit of paper tube and tape. You can get sharp photos easier if all you
have to do is lay your camera on top of a tube cut to focal length.
Try it on a telescope with self timer (no shake from button push):

that was taken with a cheap kodak with NO zoom, but i had it taped to this: 

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Raid
Hazard to Everyone
  
Posts: 201
Registered: 14-11-2022
Location: N/A
Member Is Offline
Mood: School
|
|
Nice telescope 
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Homemade crystals of manganese(II) sulfate, purified from cheap pottery MnCO3 that gave a foul reddish-black solution on acidification. Very rewarding
to make!
 
[Edited on 2-24-2023 by Amos]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I happened to have some dry ice, so I made some dinitrogen trioxide to show my students.

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 267
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
Picric Acid Recrystallization

The only yellow chemistry I'll do.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
|
|
|
Rainwater
National Hazard
   
Posts: 798
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Sunrize over the mountain, pre daguerreotype era
"You can't do that" - challenge accepted
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Neodymium sulphate octahydrate. I was rather surprised to see these- they grew from the filtrate after collecting and washing the same substance with
near-boiling water.

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Rainwater
National Hazard
   
Posts: 798
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Unknown mineral
Found this deep purple colored layer (2mm thick) in south eastern united states.
Adjacent layers are sandstone quartz mixture this lots of pyrite

Daylight
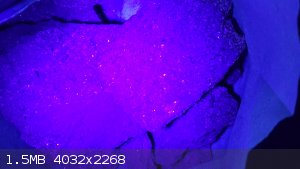
UV light
[Edited on 25-4-2023 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
Rainwater
National Hazard
   
Posts: 798
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
There be gold in them industrial controllers

Got me about 1/2 a beers worth here
"You can't do that" - challenge accepted
|
|
|
xxxhibition
Harmless

Posts: 12
Registered: 25-1-2021
Member Is Offline
|
|
Nice green Crystals.
This is CuCl2•XH2O
the dihydrate 

|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I turned 47 today. I was more busy last year helping people from Ukraine, but still had some time to synthesize some new compounds which itself made a
separate topic in my celebration which I want to share with my SM friends 

|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Happy birthday, Teodor!
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Based on the color, it looks like there is still a significant amount of HCl hanging on.
Tetrachlorocuprate gives it that lime green color. CuCl2 • 2 H2O is more of an aqua blue-green color when pure.
Recrystallizing a couple times from water should correct that.
Also, happy birthday teodor!
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Happy birthday Teodor! You're just about a year n half my senior, chronologically. I hope you have/had a great time. Looks a fair bit cooler there
than it is here this glorious day. Supposed to hit 102°F today and 108°F tomorrow. Glad you got some sciencing time, always a treat to get to paly
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Thank you, Bedlasky and Violet Sin. On the background of my picture is a frame of my home observatory by the way. I am also "staring at stars from the
back yard" when the weather permits. Yes, 102F is pretty common for Ukrainian summer for last 20 years or so (the global warming), and now I live in a
different clime.
I promise to upload some new pretty chemical pictures soon 
|
|
|
| Pages:
1
..
73
74
75
76 |