| Pages:
1
..
12
13
14
15
16
..
78 |
Thanatops1s
Hazard to Self
 
Posts: 54
Registered: 24-6-2013
Member Is Offline
Mood: No Mood
|
|
In attempting a DDNP synthesis, everything seemed to proceed as it seems to right up until the diazotization. After adding the initial H2SO4 it turned
brownish(only took a small amount too). After adding NaNO2 solution I did notice a darker brown precipitate how after filtering, the precipitate was
basically absorbed and became inseparable from the filter paper other than a miniscule amount.
I have saved all filtrates and precipitates just in case anything is salvageable.
[Edited on 7-6-2014 by Thanatops1s]
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
making pentaerythrite
It is said that the pentaerythrite is the reaction product of four mole weights of formaldehyde
and one mole weight of acetaldehyde. How much easy and possible to make some is from a home chemist ? As everybody know, pentaerythrite is difficult
to buy.
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
I agree...the PETN i got from mixed acids was full of low-/undernitrated material and after the recrystalisation it where fine pure needle-like
crystals that where easy to compless to a good density...PETN made this "pure" HNO3 seems to containvery little amounts of low-/undernitrated material
and has an completely different cystal structure...maybe the PETN from mixed acids is just the TRI-nitrate? i dont know...
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
Quote: Originally posted by underground  | It is said that the pentaerythrite is the reaction product of four mole weights of formaldehyde
and one mole weight of acetaldehyde. How much easy and possible to make some is from a home chemist ? As everybody know, pentaerythrite is difficult
to buy. |
seems to be "a bit" difficult 
*file added*
if it would be easy enough i wouldnt use any other high performace explosive...PETN just rocks (high yields, good performace, easy to handle and so
on...)
Attachment: PE-Synthese.txt (2kB)
This file has been downloaded 718 times
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by VladimirLem  | Quote: Originally posted by underground  | It is said that the pentaerythrite is the reaction product of four mole weights of formaldehyde
and one mole weight of acetaldehyde. How much easy and possible to make some is from a home chemist ? As everybody know, pentaerythrite is difficult
to buy. |
seems to be "a bit" difficult 
*file added*
if it would be easy enough i wouldnt use any other high performace explosive...PETN just rocks (high yields, good performace, easy to handle and so
on...)
|
Yes that is true. i believe it is not worth of making RDX as long as there is PETN in the game.
|
|
|
aldofad
Hazard to Self
 
Posts: 56
Registered: 30-6-2013
Member Is Offline
Mood: No Mood
|
|
Yes, I used H2SO4 to reduce water in HNO3. I'll recrystallize so
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Quote: Originally posted by VladimirLem  |
I agree...the PETN i got from mixed acids was full of low-/undernitrated material and after the recrystalisation it where fine pure needle-like
crystals that where easy to compless to a good density...PETN made this "pure" HNO3 seems to containvery little amounts of low-/undernitrated material
and has an completely different cystal structure...maybe the PETN from mixed acids is just the TRI-nitrate? i dont know... |
As far as I know the trinitrate and lower analogues are more or less water soluble and therefore removed to a considerable amount by washing and
recristallisation solutions. Although I have not found direct references to the exact solubility of trinitrate in water....
Literature gives strong evidence on the (partial) formation of the trinitrate in mixed acid nitration systems though:
Attachment: US3408383.pdf (410kB)
This file has been downloaded 572 times
Attachment: US3806578.pdf (299kB)
This file has been downloaded 546 times
Attachment: ja01608a059.pdf (301kB)
This file has been downloaded 608 times
Exact science is a figment of imagination.......
|
|
|
aldofad
Hazard to Self
 
Posts: 56
Registered: 30-6-2013
Member Is Offline
Mood: No Mood
|
|
I add a very personal question to this discussion, even the PETN formed from a not complete trinitration will be soluble in acetone and then
crystallized, isnt'it?
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Quote: Originally posted by aldofad  | | I add a very personal question to this discussion, even the PETN formed from a not complete trinitration will be soluble in acetone and then
crystallized, isnt'it? |
Yes, of course. In case of lower nitrated byproducts you should just observe a loss in yield upon recristallisation (the lower nitrated "contaminants"
left in solution).
Here's a quite informative article regarding pentaerythritol nitration in mixed acid systems:
Attachment: Nitration of pentaerythritol by HNO3-H2SO4-H2O system .pdf (1.2MB)
This file has been downloaded 619 times
Exact science is a figment of imagination.......
|
|
|
SirViking
Harmless

Posts: 20
Registered: 1-7-2014
Member Is Offline
Mood: No Mood
|
|
In my experience, you should definitely recrystallize your PETN prior to use. Recrystallization will help to filter out any impurities and, depending
on your recrystallization process, produce needle-like crystals of high surface area which can affect the detonation qualities of the HE.
[Edited on 7-7-2014 by SirViking]
If you come upon a fork in the road, take it.
|
|
|
Bert
|
Threads Merged
8-7-2014 at 16:58 |
Bert
|
Threads Merged
8-7-2014 at 16:59 |
Vpatent357
Harmless

Posts: 22
Registered: 10-7-2014
Member Is Offline
Mood: No Mood
|
|
dextrinated silver acetylide/nitrate?
Hello!
What do you think of produce SA.DS like LA by adding dextrin to acidic solution of AgNO3? before bubbling
This can make it less sensitive because SA.DS will form small crystals, bad idea?
I also consider reducing the static sensitivity by adding 1% microfine Graphite powder or 1% Conductive Carbon black...
what methods do you use?
|
|
|
Turner
Hazard to Others
  
Posts: 197
Registered: 2-12-2013
Member Is Offline
Mood: No Mood
|
|
Dextrin is not necessary in making SADS because you won't be getting large enough crystals to be of any concern. You will have a powdery substance
when dried. Not to mention SADS has lower impact and friction sensitivity than LA so it is even less of an issue.
|
|
|
SirViking
Harmless

Posts: 20
Registered: 1-7-2014
Member Is Offline
Mood: No Mood
|
|
What is the necessary water to acetone/RDX solution ratio required to recrystallize RDX?
If you come upon a fork in the road, take it.
|
|
|
Turner
Hazard to Others
  
Posts: 197
Registered: 2-12-2013
Member Is Offline
Mood: No Mood
|
|
Try adding roughly 3-4 times the volume of cold water to the solution.
|
|
|
Turner
Hazard to Others
  
Posts: 197
Registered: 2-12-2013
Member Is Offline
Mood: No Mood
|
|
Try adding roughly 3-4 times the volume of cold water to the solution.
|
|
|
Bert
|
Threads Merged
10-7-2014 at 21:54 |
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Has anyone thought of the structure N(NH2)2NO2 ?
What I am trying to present is similar to N(NO2)3 but with 2 NO2 becoming NH2.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Diaminonitramide? Can't find any references to it.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Is it alright to assume if for example, nitroguanidine exist, so most likely will nitrotetrazole?
or maybe dinitrourea, so dinitrotetrazolone?
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
http://lmgtfy.com/?q=nitrotetrazole
but i couldnt find results for dinitrotetrazolone...
i have not much knowledge of chemistry, but often things you wrote arent that logical in real life^^
example: Hexamine->Mono/Dinitrate->"Nitro"compound....how on earth make this sense lol
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
I obviously know nitrotetrazole exists, that was an example as an analogy to nitroguanidine.
Since R-NCO + R-N3 > 1,4 R tetrazolone, NaN3 + NO2SbF6 > NO2N3
and, AgOCN(s) + ClNO2 > AgCl + OCNNO2, OCNNO2 and NO2N3 could probably form dinitrotetrazolone although I really doubt it since the nitro group
usually prevent the tetrazole ring forming.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
DubaiAmateurRocketry is an account used by several different people-
Some of whom are more knowledgable than others. As I never know what knowledge level (and hence appropriate depth of an answer) is waiting to receive
on the other end of this, I've given up on answering their questions at all.
PM'd suggestions they obtain individual accounts were blown off.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Dany  | PHILOU my friend, i didn't tell you that liquids are incompressible. liquids are compressible, even solid are compressible but to a much lesser
extent. The propagation of sound in liquid compresses and decompress successively the liquid, however the compression is infinitesimally small to make
a density variation in liquid (or solids). However, under high dynamic shock wave liquid and solid will be compressed to a smaller volume. this is
obvious from the unreacted Hugoniot diagram (the P-V diagram). This phenomena is observed under several GPa of dynamic pressure. For example liquid
TNT has a density of ρ= 1.473 g/cm3 at ambient pressure. A shock wave entering the liquid with an amplitude of P=10.855 GPa (approx. 109
kbar) will compress the liquid TNT to a density of 2.259 g/cm3 [1]. of course the P-V diagram for the liquid TNT is called the unreacted
liquid TNT, so no detonation is observed (the density of 2.259 g/cm3 do not correspond to the density at the Chapman-Jouguet point because
as is already told, it is the unreacted Hugoniot). Your example of density change between sea level and the mountain peak is meaningless. Btw, you are
telling me that at ambient pressure the water will dilate between 4 and over 4000°C, the question: is water still a liquid at these temperature 
Reference
[1] LASL SHOCK HUGONIOT DATA, LOS ALAMOS SERIES ON DYNAMIC MATERIAL PROPERTIES.
[Edited on 18-6-2014 by Dany] |
You didn't tell that liquids are incompressible but you did wrote: "It is the first time that i hear that the density of liquid change with
pressure..." what means pretty much the same 
The question was if density change with altitude...not if the effect was strong or weak. True that the compressibility of gases is much bigger than
the one of liquids, itself usually bigger than the one of solids.
Most liquids will expand under reduced pressure and contract under increased pressure...some will even freeze under pressure.
I'm analysing datas on that subject to give you numbers...the effect is weak but not negligible...I think it is in the order of 0.5-1%.
"Btw, you are telling me that at ambient pressure the water will dilate between 4 and over 4000°C, the question: is water still a liquid at these
temperature  " "
Water at ambiant pressure turns into a gas above 100°C, between 100°C and 1800°C it remains molecular, but above it starts to decompose into its
elements...so water dropped onto a white heated 2000°C metallic plate will explode in a flash due to overheating of water, decomposition into H2 and
O2 and recombination into H2O. The H2O gas and later the H2 and O2 gases will continue to expand with heating.
Here follows a typical diagram of density of a substance as a function of T°. The example is TCM (tetrachloromethane) at ambiant pressure wich
display a fusion T° of -23°C and a boiling T° of 76.7°C; you can see that at the melting point there is a gap of density; but that at the boiling
point the volume variation is almost continuous.
[img]http://[/img]
Attachment: php1950XX (58kB)
This file has been downloaded 715 times
[Edited on 26-7-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by Dany  | It is the first time that i hear that the density of liquid change with pressure...the volume of liquid will increase with increasing temperature (so
density drops). water is an exception because it has a negative thermal expansion coefficient, it will contract upon heating...what will change with
altitude is the boiling temperature of the liquid because the ambient pressure decreases with altitude. However, the bulk density will remain
unchanged.
These claims PHILOU(density of liquid drops with decreasing the pressure) need to be backed up with a new theorem...
Dany.
[Edited on 17-6-2014 by Dany] |
Dany,
I don't know what is your education level, experience, work or fields of specialities... but I think your studies are far away and maybe you didn't
got physical chemistry and thermodynamic?
Maybe you are not familiar with the isothermic p(V) curves the diagram of gas-liquid near the critical point explaining the perfect gas laws and the
variation with real gas showing distillation plates (during liquefaction of gas, the pressure remains constant). I'll put you a picture.
Water has also a positive thermal expansion coefficient...it only display a singularity between 4 and 0°C!At ambiant pressure, between 4 and over
4000°C it dillates; between -270 and 0°C also ice dillates!
If liquids were uncompressible, why would sound travel through?
[Edited on 18-6-2014 by PHILOU Zrealone] |
Here is the picture from Paul Arnaud's book: Cours de chimie physique.
p184: Isothermic curves pressure-volume.
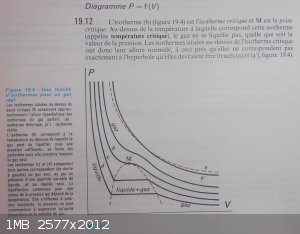
You clearly see on the isotherms below the critical point that volume variation as a function of the pressure is high on the right side of the
liquefaction plate; but on the left side volume variation is less as a function of the pressure but stil present.
Here is attached a document of the study of 18 liquids as a function of pressure and T° that clearly shows that density of liquids is not only
temperature dependant but also pressure dependant.
Attachment: The volume of 18 liquids as a function of p and T-Large.pdf (3.2MB)
This file has been downloaded 717 times
[Edited on 26-7-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
Thank you Philou for posting the textbook of P.W. Bridgman which back up which i post in my previous comment that the pressure of
liquid change under very high pressure. for example you can see (in the bridgman book) that at a given temperature the volume of liquid change very
little even at 500-1000 bar range. So let's back to the original story of liquid density variation with altitude...the rapid answer is that the
variation is practically zero et pour ne pas oublié, merci beaucoup pour me mettre la photo que ta pris du livre d'Arnaud! Je pense que l'internet
est plein de livres d'anglais (que tu peux pirater) pour copier et coller des courbes d'isothermes et autres courbes de ta première ou deuxième
année universitaires 
Dany.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
AIAA Propulsion Energy 2014 conference provide live stream on the internet. Although I havent seen much talk in EM, might be useful.
http://new.livestream.com/AIAAvideo/PropEnergy2014
|
|
|
| Pages:
1
..
12
13
14
15
16
..
78 |