| Pages:
1
..
19
20
21
22
23
..
78 |
Spartan
Harmless

Posts: 13
Registered: 26-3-2015
Member Is Offline
Mood: No Mood
|
|
I have never made silver acetylide/nitrate but i have heard that you can get around 3g (maybe a little bit more) silver acetylide/nitrate from 5g
silver nitrate. So i think your yields are ok.
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Correct Me I Iam Wrong
I cunsolted one of the senior members on this board, and I was told that no HNO3 is need, as the silver nitrate breaks down in in water and creates
HNO3, just make sure you do not have too much water so your HN03 is more concentrated.
In the book "Primary Explosives" the author states the that a hot acidic solution will yield the least sensitive SADS... so it may be good to
add a little HNO3 anyways. See the link for the book below... I have full access through a university Library, so feel free to ask more questions.
https://books.google.com/books?id=wfJHAAAAQBAJ&pg=PA305&...
|
|
|
darklight
Harmless

Posts: 5
Registered: 26-4-2015
Member Is Offline
Mood: No Mood
|
|
how about nitroglycerin
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
How about it?
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
That darn Nitro... He sooo funeee!
Oh boy! 
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | | Does anyone know what the equilibrium moisture content of AN is at say, 20C and 65% RH? I'm asking because I have a lot of AN in solution (from CAN
fertilizer) and want to recover it. I know I could just heat it to drive off the water, but I would like to avoid spending more electricity than
necessary. |
Found the following which is from an old department of agriculture technical bulletin (912) from the set "Technical Bulletin, Issues 901-915". The set
of technical bulletins can be downloaded from Google books. The section on ammonium nitrate and moisture content is attached. It also has a lot of
information on several other nitrate salts as well as other chemical fertilizers.
"A material is said to be hygroscopic when it absorbs moisture from the air at ordinary temperatures and humidity. This occurs when the pressure of
water vapor in the air exceeds that of the saturated solution of the material."
Attachment: Ammonium Nitrate Moisture Content Technical Data.pdf (556kB)
This file has been downloaded 561 times
 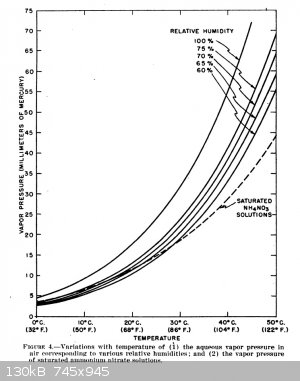
I remembered seeing the following from the archive "Yarchive" which is regarding the same concept as above for determining when a material will absorb
water from the air (or when it will lose water to the air or dry).
"
From: glhurst@onr.com (Gerald L. Hurst)
Newsgroups: rec.pyrotechnics
Subject: Re: KNO3+S+C/Saltpeter(even more;-)
Date: 16 Nov 1995 21:14:25 GMT
Organization: Consulting Chemist
Lines: 110
In article <1995Nov16.152055.17452@cs.rochester.edu>,
nelson@cs.rochester.edu (Randal Nelson) says:
>>>>There's a nice exercise for you budding chemists out there. Erik
>>>>has opined that NaNO3 becomes hygroscopic at about 73% (?)
>>>>relative humidity. How about checking this figure using only
>>>>solubility data and comparing the result with similar calculations
>>>>for KNO3. In other words, see if you can figure out the highest
>>>>relative humidity at which you would expect black powder made from
>>>>the two materials to remain stable at say 0 deg and 30 deg C.
>
>>Calculations like this are naturally subject to errors arising
>>from the nonideality of strong solutions, so how would you
>>check the results experimentally using only materials you can
>>buy over the counter, not including a hygrometer?
>
>>>>OK, I'll furnish the solubility data:
>>>>
>>>>Soly in g of KNO3 in 100 g H2O is 13.9 (0 deg), 21.2 (10 deg),
>>>>31.6 (20 deg) and 45.3 (30 deg).
>>>>
>>>>The corresponding values for the sodium salt in g are:
>>>>
>>>>73.0 (0 deg), 80.8 (10 deg) , 87.6 (20 deg) and 94.9 (30 deg)
>>>>
>>>>Jerry
>
>OK, since I havn't seen anyone take a shot at this.
>
>You can make a first approximation based on the molar fractions
>of the ionic solutions. For example, take NaNO3 at 20 degrees.
>100 gm of water is 100/18 = 5.56 moles. 87.6 gm of NaNO3 is
>87.6/85 = 1.03 moles, which in solution is twice that number, or
>2.06 moles of ions. The molar percentage of water is then
>5.56 / (5.56 + 2.06) = 72.9%, which, to a first approximation is
>the vapor pressure as a fraction of the 100% relative humidity pressure,
>and hence the humidity at which a saturated solution will pull moisture
>from the atmosphere. This agrees with Erik's figure.
>
>For KNO3, 31.6 gm is 31.6/101 = .313 moles = .626 moles of ions,
>giving a molar fraction for water of 5.56/(5.56+.616) = 90.0%.
>Pretty sticky conditions.
YES! Very nicely done, and probably not too far from reality.
Sodium nitrite has a slightly higher molal solubility than
the nitrate and is known to maintain RH at 66 percent
@ your 20 deg, versus 69.5 percent calculated by the method
you used. We might expect the value for KNO3 to be even more
accurate because of the lesser solubility.
Your answer explains why NaNO3 is not as well suited for
commercial black powder. Although the relative humidity
occasionally climbs over 90 percent in some parts of the
country, it spends a lot more time under this figure. If
BP is anything like AN then it probably dries out at least
as fast as it gets wet from atmospheric moisture and
that means your powder is probably going to be dry when you
go to use it unless your timing sucks.
70 percent humidity is another scene entirely. In the Dallas
area, the humidity tends to rise every summer evening to well
over 60 percent so that if you have spilled AN around, it
will be in liquid pools every morning when you come to work,
but dry out by mid morning and then reliquify as you're heading
home. SN is just about 10 RH units better, so it also spends
a fair portion of its time absorbing moisture, if not in
Dallas, then in Houston  If you happen to live in Reno, If you happen to live in Reno,
then you could probably get away with an AN-based powder,
at least most of the time.
As a matter of mainly academic interest, it might be noted
that the solubility of KNO3 increases more rapidly with
temperature than does that of NaNO3. The steeper slope
of the curve means that difference in the hygroscopicity
of the two salts grows narrower at high temperatures and
both salts become increasingly deliquesent, i.e absorb
moisture at lower RH.
If you want to determine the exact RH at which KNO3 and
NaNO3 become deliquescent, the easiest method would be to
put an excess of the well wetted solid salt in a jar
with much a much smaller weighed quantity of either
carefully dried CaCl2 or a strong aqueous solution
of known concentration of CaCl2. THe CaCl2 will slowly
absorb moisture from the wet nitrate, forming a solution
which becomes more dilute with time until equlilbrium
is reached. If the nitrate is still wet at this point,
the solution above it must must have the same vapor
pressure (i.e. the same RH) as the now diluted CaCl2
solution, and that value can be obtained by calculating
the CaCl2 concentration from the weight gained by H2O
absorption and looking up the corresponding RH in the
convenient tables of such values in the CRC or Lange's
handbook.
There are also tables for H2SO4 and NaOH, but I originally
suggested that the materials could be purchased "over-
the-counter" and I now suggest that CaCl2 may be a bit easier
and safer to purchase and handle. There are any number of
other common substances with known solution vapor pressures,
but you might have to look farther than he CRC to find them.
Lest you accuse me of being too academic (or as Beavis might put
it: "Fancy Schmantzy"), allow me to mention that I have used the
method outlined above to determine the parameters for building
an explosives manufacturing facility (using H2SO4) and to
gather data on emulsion explosives for a patent battle between
a couple of major corporations. In both cases, I employed Mason
jars for the experiments. The plant (Kinepak) worked fine, and
we won our case 
Jerry"
[Edited on 28-4-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
2 Ag+ + HCCH --> Ag2C2 + 2H+, so the HNO3 is formed by the reaction anyway, you only need a little to promote complex formation. IIRC, there are
more complexes of Ag2C2/AgNO3, something like AgC2*AgNO3 but also AgC2*6 AgNO3. Maybe hot and more acidic solutions promote the latter? Would have to
check PATR again...
[Edited on 29-4-2015 by nitro-genes]
|
|
|
Bert
|
Threads Merged
29-4-2015 at 15:39 |
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Non-Ketone Organic Peroxides
Can anyone shed some light on the possibility of making organic peroxides from substances other than ketones (acetone, MEK, etc.), such as from
alcohols or aldehydes? I am aware that HMTD is an organic peroxide, but are there more accessible chemicals or fuels that can be made into peroxides?
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Here is a site that lists some peroxide forming chemicals.
http://blink.ucsd.edu/safety/research-lab/chemical/storage/p...
http://safetyservices.ucdavis.edu/snfn/safetynets/snml/sn23/...
[Edited on 6-5-2015 by Loptr]
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Wow, this is exactly what I was looking for  Thank you sincerely Loptr Thank you sincerely Loptr
|
|
|
Bert
|
Threads Merged
6-5-2015 at 07:03 |
smithdotyu
Harmless

Posts: 20
Registered: 9-2-2015
Member Is Offline
Mood: No Mood
|
|
I made tetrazene,but it looks like not sensitivity for hammer hit
Hi all;
I made some tetrazene use Aminoguanidine sulfate + NaNO2 。
I used phosphoric acid to catalyst.make the PH at 5~6 and the temperature
is 50C。
finally I get 1gram tetrazene, and wait it dry,it's orange color,I try to burn it,it burning not fast like HMTD or NHN,slow and make sounds
like ’pa、pa、pa‘
In the book they sad tetrazene is sensitive,but i use hammer to hit it,it not explosive 。should I would add some KClO3 to it?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
You have most likely obtained guanyl azide ─ a consequence of the presence of mineral acids in the reaction mixture!
Take a long look at Davis's prep. in COPAE?
|
|
|
Bert
|
Threads Merged
18-5-2015 at 06:27 |
havenochoice
Harmless

Posts: 1
Registered: 31-5-2015
Member Is Offline
Mood: No Mood
|
|
Ok, dont slam, newbie to the scene needs help
Ok, first off, i am new here and the reason for my post is confusion!
I got the bug because i made my first ever batch of black powder last week from materials purchased, and it actually worked!!!!
I am just an individual and not a university of chemical engeneer so i cant get access to other chemicals only those that are buyabale by the general
public.
Black Powder:
75% Potassium Nitrate ( got online from the USA, havent got a clue how it got through customs as you cant buy it here! ).
15% Charcoal.
10% Sulphur.
Now here is where my problems begain.....
I tried to create some AP, i know your all against this but it was the only thing i could make as other materials are not allowed to be purchased
here!
So....
I had Acetone, Hydrogen Peroxide (9% strength as its the highest strength you can get here, i searched for the 30% but just could not get any ), HCL
(33%), and lastly Sodium Bicarbonate.
I mixed the Acetone & HP, cooled it to 4C and then slowly added the HCL catalyst keeping it bellow 8C. I waited a few hours then mixed in the
Sodium Bicarbonate to neutrualize the reaction....So far so good,BUT, then a few hours later my eyes started streaming and wouldnt stop, i am guessing
the acid had befome gasous. Now i am let with a slimy talc like substance.....I tried exploding a 3mm square sample via a rocket hobbyists fuel cap
but the AP did not ignite. Have i ruined the mixture somehow? or will this become combustable once dried? Should i allow it to become dried out?
I also tried to make some ANFO or rather tried to yet again buy ingrediants to make ANFO but i can not get Amonnium Nitrate no matter where i go, so
instead i managed to get Urea and some disel as i read Urea could be used instead of Amonnium nitrate.BUT, once again this would not detonate....
In essence i am asking would AP (primary) UFO (secondary) detonate? Or am i missing something obvious to eveyone else and not myself because i am too
close to see it?
Will the AP be safe to allow to dry out, and will it then become explosive?
And would UFO work or have i missed something again, maybe Nitric Acid, but that acid is impossible to get, could something other be used that
actually is purchasable ( god only knows what that would be it seems everything is illegal! )...
Ps... i am not a mad terroist, i am actually just some one who thinks knolage should be avaliable to everyone and why shouldnt people be allowed to
know everything? Its only if they then use that knoladge for illegal gains should they be rightly arrested, not just for wishing to expand they
mind....Sorry for the speech, just sick of being accused of being bad just because i believe in freedom of knowladge!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
ANFO is not easy to detonate.
You need confinement and good detonator ...wet CTAP (cyclotriacetonperoxyde) will certainly not do the job.
Urea may be subtituant for Ammonia but then you need to nitrate it to get Urea nitrate (UN).
So UNFO may replace ANFO to get your UFO to fly  and that's an INFO and that's an INFO  . .
UNFO will also need a good confinement and detonator.
There is no need for sodium bicarbonate to neutralise the CTAP...simply filter and wash with cold water.
The bicarbonate may have induced some decomposition of your CTAP yielding chloroaceton...reson why you cried ... yes it is a lacrymator.
Due to your lack of knowledge and chemical education I really think you should stick to black powder...you will arm yourself or others trying to play
with CTAP at this stage.
Knowledge is the power, but to be knowledgeable, you need to read and understand fully what you are playing and dealing with.
First read, then read ... and read even more. Start small and never think you masterize the stuff...it will always remind you one day or another who
is the real boss...it's statistical and darwinian so always imagine the worst case scenario...the stuff exploding or detoning right now.
[Edited on 31-5-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Philou is right good confinement and a decent detonator, even a booster maybe for ANFO and dry AN before mixing.
Urea nitrate is annoyingly quite hygroscopic and can be detonated on its own without fuel oil fairly easily.
I don't know what happened with your AP synth, it sounds correctly done and shouldn't be that hard to mess up.
I would definitely use the Sodium bicarbonate wash especially if using HCL as catalyst just because it would remove all traces of residual acid in the
final product which you don't want in something like that (decomposition/increased sensitivity) but that's just me.
It is less sensitive when wet but not useable until dry really.
I agree with PHILOU, you should keep to the BP for now til you have read ALOT of information on energetics and understand how they work and their
properties. Not knowing what your doing, especially when trying to manufacture and use something sensitive like AP, could end very badly as sensitive
primaries are pretty unforgiving if you treat them the wrong way.
[Edited on 31-5-2015 by greenlight]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Urea nitrate is not hygroscopic, but it does require careful washing and drying after synthesis from what I have seen to avoid decomposition. AP is
quite volatile and not a terribly efficient initiator, but I doubt it is out of the range of normal sensitivity as compared to the primaries in common
usage . I would always try to err on the side of caution though and not make assumptions about sensitivity and keep the quantities low.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
If you've obtained potassium nitrate already, all your are missing is some sulfuric acid that you can use to make much more stable and reliable
explosives, such as ETN, Picric Acid, and nitrocellulose.
|
|
|
Bert
|
Threads Merged
31-5-2015 at 11:00 |
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Wow I thought Un was hygroscopic.
I made it when I was very young and I remember it was a bitch to get 100% dry. Maybe I hadn't washed it enough, it was so long ago I forgot what I
did.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Yeah, it is not hygroscopic from what I remember. However, from what I have witnessed it easily decomposes if much heat is used in attempts to dry it
and especially in the presence of acidity which catalyzes the decomposition. When UN decomposes it produces nitric acid which catalyzes further
decomposition. It may seem when heating UN that it is very hygroscopic, and that it will never dry, but it is the presence of acidity and the water
the acidity strongly holds onto that keeps it wet. It will become seriously degraded before it ever dries out if anything more than very gentle
heating is used to dry it, because it will simply keep decomposing. UN that has been significantly decomposed by aggressive drying techniques
involving heat has lousy explosive properties also, speaking from experience. I would advise washing the acidity from the crystals as much as possible
after synthesis with ice cold water, or some other solvent that the UN is only slightly soluble in, and then blottering away the moisture as much as
possible by placing the crystals on a stack of absorbent cloth of some kind before using moving air and no excessive heating to dry the material.
[Edited on 1-6-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Antoine
Harmless

Posts: 13
Registered: 3-6-2015
Member Is Offline
Mood: No Mood
|
|
AN/AL with not fine AL powder
Hello! The aluminium powder would to be fine to make AN/AL?
I make my powder with a piece of aluminium and with a sheet of fine sandpaper.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Yes, the finer the better.
If your using small particle sandpaper and it is coming out like dust you should be good.
Grind the AN so it is fine as well and make sure it is dry before mixing.
[Edited on 4-6-2015 by greenlight]
|
|
|
Bert
|
Threads Merged
4-6-2015 at 03:58 |
Antoine
Harmless

Posts: 13
Registered: 3-6-2015
Member Is Offline
Mood: No Mood
|
|
Yes, it´s a fine dust of aluminium. Thanks you!
|
|
|
Antoine
Harmless

Posts: 13
Registered: 3-6-2015
Member Is Offline
Mood: No Mood
|
|
AN/AL détonation with tatp
|
|
|
Antoine
Harmless

Posts: 13
Registered: 3-6-2015
Member Is Offline
Mood: No Mood
|
|
Sorry, I send my message but I don´t finish it
So, CAN I detonate a 100g AN/AL charge with 1g of tatp in copper pipe?
Thanks! 
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
You can edit message in top right corner..
You should post these types of Q's in the short question/quick answer thread in energetic materials section.
I think 1 gram AP in metal tube should ensure detonation.
|
|
|
Bert
|
Threads Merged
4-6-2015 at 05:14 |
| Pages:
1
..
19
20
21
22
23
..
78 |