| Pages:
1
..
56
57
58
59
60
..
78 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by JJay  | What do people typically use for scrubbing nitrogen oxides when making nitric acid? Will sodium carbonate solution work, or do I need something
stronger?
|
H2O2 and Na2CO3 (or NaHCO3) should prove to be usefull...
The H2O2 will oxydise the NO (colorless) to NO2 (yellow) and the HONO to HONO2 (nitous acid to nitric acid).
2 NO2 + H2O --> HONO2 + HONO
So in fine with NaHCO3 or Na2CO3 you end up with recoverable NaNO3 and H2O and gaseous CO2...
Lately I have used HNO3(69%) and metallic Ag... you get some NxOy...
The use of H2O2 discolored immediately all NxOy color from the liquid reactant, it bubbled some O2 and accelerated a bit the very slow dissolution of
the 99,99% Ag wire.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by kratomiter  | | Thank you for the useful patents! I see that isolating pure methylamine perchlorate will be difficult, maybe I should stick to the well known
guanidine perchlorate or give a try to gel explosives. |
The patents mentions only the nitrate variant not the perchlorate one...
But:
1) It is low yielding at the 100°C used and it is dangerous... because exothermic (into the mix you have a lot of unreacted formol, some formic acid
and HNO3)....
2) The evaporation/concentration steps are not easy to get rid of water and of the formed formic acid...
3) The isolation step is not easy aswel if you want to isolate the interesting compound that is methylamine...
==> Anyone that has mixed 37% formol with 69% HNO3 knows that runnaway occurs within a few seconds...
The all batch boils into toxic NxOy fumes and accrid / toxic / carcinogenic CH2=O / HCO2H / CO2 / vapour cloud...
So better stick to the safe procedure from NH4Cl and formaldehyde whatever the form... then isolate the methylamine/ dimethylamine and
trimethylamine...
Then do the desired salt by conventionnal neutralisation with dilluted acid-base solutions and cristallization by slow evaporation (eventually helped
by a precipitating solvent like methanol, isopropanol or ethanol).
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Bert
|
Thread Pruned
9-11-2017 at 07:49 |
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Ammonium picrate has a poor oxygen balance (-52).
By mixing it with an oxidizer, will it improve its brisance and VoD?
Assuming that I'll use ammonium nitrate which has an OB of 20, how much should I mix?
Should I simply mix the powdered compounds together?
Which explosive would perform better if I use the same amount for both charges: Ammonium picrate mixed with ammonium nitrate to give a balanced OB or
ammonium picrate alone?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by joseph6355  | Ammonium picrate has a poor oxygen balance (-52).
By mixing it with an oxidizer, will it improve its brisance and VoD?
Assuming that I'll use ammonium nitrate which has an OB of 20, how much should I mix?
Should I simply mix the powdered compounds together?
Which explosive would perform better if I use the same amount for both charges: Ammonium picrate mixed with ammonium nitrate to give a balanced OB or
ammonium picrate alone? |
The mix will be better...
NH4NO3 --> N2H4O3 --> N2 + 2 H2O + 1/2 O2
NH4ClO4 --> 1/2 N2 + HCl + 3/2 H2O + 5/4 O2
NH4OC6H2(NO2)3 --> C6H6N4O7 --> 3 H2O + 2 N2 + 2 CO2 + 4C
You have to burn 4 C atoms per Ammonium picrate molecule to get the best energy output out of the mix...
This means you need 8 O atoms to make 4 CO2...
==> You thus need 4 times the first equation...
so a molar ratio 1/4 (AmPi / AN)
==> You need 3,2 times the second equation
so a molar ratio of 1/3,2 (AmPi / AP)
The mix would even be better performing with an OB balanced hydrazinium nitrate, hydrazinium perchorate or hydroylaminium nitrate or perchlorate...
The perchlorate variants will always be superior in performances to their nitrate brothers because:
-the density increase is higher,
-the sensitivity towards initiation is higher,
-the energy output per volume will be higher.
I suspect detonic parameters (VOD, brisance) to be into the following order HAP > HP = HAN > HN > AP > AN.
AN will reach the 8-8,4 km/s VOD
AP will reach the 8,4-8,9 km/s VOD
HN will reach the 8,9-9,3 km/s VOD
HP/HAN will reach the 9,3-9,5 km/s VOD
HAP will reach the 9,5-10 km/s VOD
Into such binary mixes of negative OB and positive OB... the resulting mix is always better performing than each explosive appart because each
compound provides the best energy output by using at best excess oxygen and excess fuel... what would otherwise be spectator of the detonation wave
and only burn (if a fuel) outside the detonation zone...thus later and slower.
A simple mixing will do... but like any binary mixes...the more intimate the mixing, the best...
[Edited on 13-11-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rocinante
Hazard to Others
  
Posts: 121
Registered: 13-11-2017
Member Is Offline
Mood: No Mood
|
|
If you have a SA.DS/ETN blasting cap with 2 cavities, one central with SA.DS and the other with ETN, which material for the inner tube would be better
- 0.3 mm aluminium or 1-2 mm thick plastic (polypropylene)? I'm assuming that the outer tube is made out of paper of plastic.
I'm thinking about combinaition of factors. Will the aluminium fragments present more danger than the 5-7× thicker plastic ones? (this assumes two
layers of thick gloves, two layers of face protection, blast mitigation device during assembly). What is the comparative danger of the inner tube
breaking during handling? Are there any static electricity considerations with the setup..aluminium/ETN/plastic as opposed to plastic/ETN/plastic or
aluminium/ETN/aluminium? I assume that the ETN around the inner tube is only veery lightly pressed, so some amount of material flow can happen and
static dicharges during assembly are of no concern (blast mitigation device). I also want to know if there is a significant difference in the amount
of primary needed, i.e. 150 mg of SA.DS with the aluminium tube vs. 350 mg for the thicker PP one. Also, how quickly will the 0.3 mm Al fragments lose
velocity ... is 30 m enough for it to slow down enough from ~ 2 km/s to only ~ 200 m/s?
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by joseph6355  | Ammonium picrate has a poor oxygen balance (-52).
By mixing it with an oxidizer, will it improve its brisance and VoD?
Assuming that I'll use ammonium nitrate which has an OB of 20, how much should I mix?
Should I simply mix the powdered compounds together?
Which explosive would perform better if I use the same amount for both charges: Ammonium picrate mixed with ammonium nitrate to give a balanced OB or
ammonium picrate alone? |
The mix will be better...
NH4NO3 --> N2H4O3 --> N2 + 2 H2O + 1/2 O2
NH4ClO4 --> 1/2 N2 + HCl + 3/2 H2O + 5/4 O2
NH4OC6H2(NO2)3 --> C6H6N4O7 --> 3 H2O + 2 N2 + 2 CO2 + 4C
You have to burn 4 C atoms per Ammonium picrate molecule to get the best energy output out of the mix...
This means you need 8 O atoms to make 4 CO2...
==> You thus need 4 times the first equation...
so a molar ratio 1/4 (AmPi / AN)
==> You need 3,2 times the second equation
so a molar ratio of 1/3,2 (AmPi / AP)
The mix would even be better performing with an OB balanced hydrazinium nitrate, hydrazinium perchorate or hydroylaminium nitrate or perchlorate...
The perchlorate variants will always be superior in performances to their nitrate brothers because:
-the density increase is higher,
-the sensitivity towards initiation is higher,
-the energy output per volume will be higher.
I suspect detonic parameters (VOD, brisance) to be into the following order HAP > HP = HAN > HN > AP > AN.
AN will reach the 8-8,4 km/s VOD
AP will reach the 8,4-8,9 km/s VOD
HN will reach the 8,9-9,3 km/s VOD
HP/HAN will reach the 9,3-9,5 km/s VOD
HAP will reach the 9,5-10 km/s VOD
Into such binary mixes of negative OB and positive OB... the resulting mix is always better performing than each explosive appart because each
compound provides the best energy output by using at best excess oxygen and excess fuel... what would otherwise be spectator of the detonation wave
and only burn (if a fuel) outside the detonation zone...thus later and slower.
A simple mixing will do... but like any binary mixes...the more intimate the mixing, the best...
[Edited on 13-11-2017 by PHILOU Zrealone] |
Very interesting.
Is Amatol OB balanced? If it is, why does it have such low VoD and brisance?
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I believe amatol is oxygen balanced, that is the whole reason for its design as well as to extend the amounts of TNT used.
TNT is very underoxidized on its own and this is shown with the black smoke that is visible after detonation. Ammonium nitrate is overoxidized so
making a mixture of the two brings the balance closer to 0.
I dont think the VOD is too bad considering it contains the AN which would slow it down a bit.
I can't remember it I will have to have a look when I get home.
EDIT: ANFO 5.8% fuel oil detonation velocity @ 0.82 = 4.55 km/s
AN/TNT 50:50 detonation velocity @ 1.58. = 5.97 km/s
TNT pure @ 1.58. = 6.88 km/s
According to explosives engineering.
It definitely increases the vod a lot mixing the two and thats only in a 50:50 mix which would still be underoxidized.
Could not find exact data for 80:30 or 70:30 mixtures though.
[Edited on 14-11-2017 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Rocinante
Hazard to Others
  
Posts: 121
Registered: 13-11-2017
Member Is Offline
Mood: No Mood
|
|
TNT acts as a thermobaric explosive, Waldemar A. Trzciński measured almost the same positive pressure impulses for RDX/wax 94:6 and TNT. The soot is
slightly useful. That's likely why you see TNT eq. of explosives like H-6 cited like only 135 % or 150 % for newer EBX compositions.
[Edited on 14-11-2017 by Rocinante]
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
The mixture of just RDX and TNT is not thermobaric.
The Australian C6 composition certainly on the on the edge of thermobaric with the 20%^ Al powder to increase the intensity and heat of the blast
wave.
I think the higher vod comes from the large content (^40%) of RDX which is a better performing explosive than TNT.
The thing that makes me think C6 is not a full thermobaric is the fact it is used underwater and has replaced ?torpex? Which I forgot the composition
of.
True thermobarica do not function well underwater due to lack of atmospheric oxygen.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: | | True thermobarica do not function well underwater due to lack of atmospheric oxygen. |
Aluminum (which torpex has a high % of, along with TNT and RDX) reacts just fine with the Oxygen from water vapor, CO and CO2 inside the big bubble
formed by an underwater explosion.
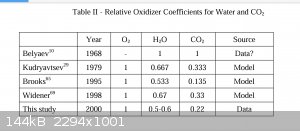
Thermobaric? Not as I generally see the military use the term, but it DOES make use of environmental Oxygen to after burn a fuel and prolong the
pressure pulse. Just not the free O2 in air.
Attachment: Summary of Aluminum Powder Testing.pdf (853kB)
This file has been downloaded 510 times
[Edited on 15-11-2017 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I didn't even think of that Bert.
I was always under the impression that thermobarics needed atmospheric oxygen to function properly to develop full pressures.
It must perform quite well underwater in fact if it is used in a formulation especially for torpedoes. Probably due to the extended pressure pulse
being good at putting holes in submarines and boat hulls.
Thanks for sharing.
[Edited on 15-11-2017 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Rocinante
Hazard to Others
  
Posts: 121
Registered: 13-11-2017
Member Is Offline
Mood: No Mood
|
|
aluminized explosives tend to burn about 2/3 of its aluminium in its gases (obviously not that great) and only about 1/3 in surrounding air, water
vapor is good, though.... underwater charges used aluminium since WWII at the latest, even now they use HTPB/AL/AP/RDX-HMX or TMETN/PCP/Al
formulations
within the art there is distinction behind thermobarics, thermobarics that burn most of their aluminium in the air are called thermobarics (TBX),
thermobarics that burn most of their aluminium in combustion gases from the nitramine fill are called EBX, enhanced blast explosives. Typical TBX is
IPN/Mg 60:40, typical EBX is PBXIH-18 (65 % HMX, 5 % binder, 30 % Al)
FAE are two stage events with mostly ethers, epoxides that are dispersed into the air and detonated via secondary charge (simple iginition won't make
DDT that easily)
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Failure modes. Not always the scenarios we assume immediately off the cuff.
Aside from the increase in total energy and pressure pulse duration being even harder on air filled things that tons of shock driven, incompressible
water hit?
The way to certainly and quickly kill a ship of ocean going size is not usually to punch a hole (water tight compartments, redundant systems, dammage
control trained crews and a really BIG structure with lots of reserve bouyancy) but to break it in two. A bigger bubble does this better-
First lift the ship really hard near the middle via an explosion underneath it, not even needing to be in contact with the hull. Then drop it back
down onto the remains of the bubble/highly gassed water where the explosion was, which now provides very much less bouyancy due to low density- Ship
will be unsupported in the middle, while normal density water supports the ends as per design. SNAP!
Happens to ships naturally sometimes too, when bow and stern are bridged across 2 sufficiently tall and widely spaced waves, leaving the midship
insufficiently supported.
[Edited on 15-11-2017 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Rocinante
Hazard to Others
  
Posts: 121
Registered: 13-11-2017
Member Is Offline
Mood: No Mood
|
|
it took about 100 kg of TNT exploding 6 m from the hull of a WWII submarine to kill it, submarines were and are hard targets (hulls designed to
survive high pressures), so the hunt for powerful explosives was fully justified
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Interesting... So shaped charges would be no good against large boats and submarines then.
I am guessing after the positive pressure pulse/shockwave and gas bubble vacuum created by the explosion there would also be a negative pressure
effect as the water rushes to fill the large void too. This would also contribute to damage to the vessel surely.
Like a violent up, down, and then back up motion to the middle of the boat/submarine.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by Rocinante  | | it took about 100 kg of TNT exploding 6 m from the hull of a WWII submarine to kill it, submarines were and are hard targets (hulls designed to
survive high pressures), so the hunt for powerful explosives was fully justified |
Or you could use that HE in a few Hedgehog projectiles
By the end of the war, navies got more sophisticated about both submarine detection and application of high explosive solutions to the submarine
problem. Less than 20 kg of HE applied directly to the hull as a shaped charge with an air chamber in front of the cone to allow it to invert properly
did the job. Getting the charge where it needs to be beats brute force.
My uncle spent a lot of WWII in a submarine, mostly Pacific theatre. He didn't talk about it much.
[Edited on 16-11-2017 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Sandman3232
Harmless

Posts: 31
Registered: 27-11-2017
Member Is Offline
Mood: No Mood
|
|
Anyone know if there is a thread about trinitrobenzene? I've been trying to find one with no sucess. And I cant seem to find much on the internet
about it either.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
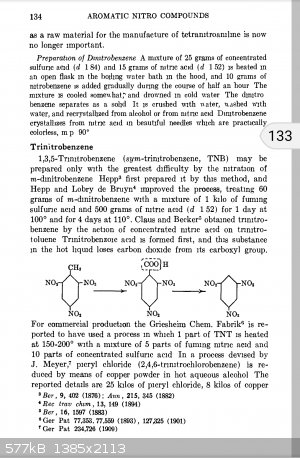
There is a preparation for TNB on Tenney Davis COPAE, to be found in sciencemadness.org library. Data on performance, sensitivity & etc. are given
as well. Read chapter 4 FROM THE BEGINNING for a complete explanation on WHY it requires harsher conditions to synthesize and general comparisons of
nitro aromatics. TNB article starts pp. 134
Tenney Davis COPAE: You should LOOK HERE before chasing non commercial stuff on Google... Which exists to put adds in front of eyeballs and harvest
metadata, not to help little old you actually find stuff out!
More expensive to make in an industrial setting than TNT and has different handling and loading characteristics which make it less attractive from
military and commercial point of view, despite somewhat higher VOD and oxygen ballance. More toxic too.
Never made it. Don't know of anyone commercially doing so.
[Edited on 30-11-2017 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
As far as I can remember TNB is not easily made in an amateur setting. It requires long nitration time and higher temperatures plus obscure nitration
agents.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
According to Davis, it was easier to synthesize TNT and demethylate that than do the brute force tri nitration of benzene itself.

Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
A kilo of fuming sulfuric and half a kilo of 99% nitric per 60 grams of dinitrobenzene!
Over a day at 100C!
It sure takes a lot of bullying to hang that last NO2 group on there.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
storing sodium lithium metal in sunflower oil ?
Can i store sodium and lithium metal in sunflower oil ? I cant find anywhere mineral oil so i was wondering if just sunflower oil will work. Also do
i have somehow to dry it from water or it is not necessary ?
[Edited on 16-12-2017 by underground]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I wouldn't suggest storing alkali metals in any animal or vegetable fats or oils, since they may react, but storing alkali metal under oils is surely
better than leaving it unprotected.
The alkali metal will dry mineral oil more effectively than just about anything else... it wouldn't hurt to dry the oil first but this is not
necessary for most purposes.
I usually buy mineral oil in the laxative section of the drugstore. You want the kind without any additives.
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Mineral oil from drugstores here cost about 15€ per 150ml....
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
It's usually a little less than that here, but I have found it for less than $3 USD per 473 mL shopping around.
|
|
|
| Pages:
1
..
56
57
58
59
60
..
78 |