| Pages:
1
2 |
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Methods of Chlorination of Primary non Benzylic Alcohols
I have been looking for a method to chlorinate a primary amino alcohol,aliphatic arene non benzylic. Other than using thionly chloride and the various
PX,X=halogen, .
It has been suggested to me to use the Lucas test reagent, ZnCl/HCl , with low yields, and also a combination including cyanuric acid.
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Clarify this question for me please. You want to replace the OH group with a Cl in a molecule with a NH2 group?
I was under the impression the whole point of the Lucas test was that nothing happened with primary alcohols, thus telling them apart from secondary
alcohols (slow reaction) and tertiary alcohols (rapid in the cold).
What stops your C-chloro amine being stable at all, why wont it attack itself forming secondary or higher amines?
Edit,
If you want an N chloro molecule the alcohol wouldnt interfere, youd just use HOCl which could be made in situ.
[Edited on 25-10-2004 by Marvin]
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Ref: chlorination of alcohols
The question is a method to chlorinate the amino alcohol
C6H5-CH2-CH(NH2)-CH2OH (Phenylalaninol)........solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
svm
Harmless

Posts: 31
Registered: 21-7-2004
Member Is Offline
Mood: No Mood
|
|
acetic acid + Cl2 might work.
could displace the alcohol and/or the amine though.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Chlorine......for chlorinating tertiary alcohols
Remember that primary alcohols are the hardest to chlorinate, so while it's true that Cl2 will chlorinate tertiary alcohols , it will not do the
job with non benzylic primary alcohols. Routinely Thionyl chloride and other Phosphoric type haloganated acids are the main stay for this process. I
however want to avoid these hard to get acids. Also in a highly acidic environment the amine group will not react such is the case with the afore
mentioned acids.
I have recently seen much use of the sodium borohydride with Aluminum(111) as a viable method to reduce this stubborn OH group to an alkane. However
the method has not been tried.
I want to stay with some OTC method with easily available material. ....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
svm
Harmless

Posts: 31
Registered: 21-7-2004
Member Is Offline
Mood: No Mood
|
|
sorry, I thought you were looking to chlorinate the aromatic ring. my bad.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Ref: chlorination of alcohols
Well it seems that the use of ZnCl + HCl will work on the chlorination of alcohols , known as the Luca's test which will chlorinate.....
tertiary> secondary> primary and of course benzylic aromatic alcohols.
It has been mentioned by a good source at The- Hive that the so called chlorinating test will also chlorinate
primary non benzxylic aromatic alcohols with improved yield if some heat is applied to help the reaction.
...............solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Yes the chlorination of alcohols by ZnCl and HCl works, but its not the HCl and not the zinc-chloride which does the job.
The concentration of HCl has to be high enough then a complex zinc-chloro-acid is formed which actually chlorinates.
This says dry zinc-chloride and 37% HCl to has be used. Alternativly ZnO (available as white pigment for house-painting) is dissolved in muriatic acid
of 30% concentration. Then HCl-gas is bubbled through this solution in the cold to get an as high as possible concentration of HCl again. Bubbling
HCl-gas through the reaction mixture whilst chlorinating the alcohol might be a good idea.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Ref: chlorination of alcohols
Here is an interesting article on chlorination of alcohols by using TCT/DMF......solo
--------------------------------------------------------------------
An Efficient Route to Alkyl Chlorides from Alcohols Using the Complex TCT/DMF
Lidia De Luca, Giampaolo Giacomelli,* and Andrea Porcheddu
Organic Lettes 2002, vol.4,no.4,553-555
Abstract
Efficient conversion of alcohols and â-amino alcohols to the corresponding chlorides (and bromides) can be carried out at room temperature in
methylene chloride, using 2,4,6-trichloro[1,3,5]triazine and N,N-dimethyl formamide. This procedure can also be applied to optically active carbinols.
Note: Leggo originally introduced the article at the hive .....credit where credit is due
[Edited on 24-11-2004 by solo]
Attachment: Efficient method going from OH to Cl.pdf (54kB)
This file has been downloaded 2721 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 294
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
Wow, that's a real good finding, especially as TCT (TCCA) is OTC available. If they don't already know it, you should post this on the hive,
when they're online again.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Ref : Chlorination of Primary alcohols non benzylic arene
I found this patents for which chlorination occurs by different methods......
Here are a few things that I found
Patents: GB418043 Process for the Chlorination of Hydrocarbons
GB570374 Chlorination of Unsaturated alcohols
US3290395 Chlorination of Unsaturated alcohols in the Substantial absence of water and in the Presence of Hydrogen Chloride. I will include the pdf of
this one which seems the most interesting , ..
I will read through this, there may lie an gem within......solo
[Edited on 10-12-2004 by solo]
Attachment: chlorination of unsaturated alcohols without water and HCL gas....pdf (242kB)
This file has been downloaded 1829 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Ref: chlorination of alcohols
A Systematic study of the Preparation of Alkyl Chloride from the Corresponding Alcohols
R.H.clarke , F.R.S.C. and H.R.L.Streight
Attachment: a systemic study of the preparation of alkyl chlorides from the corresponding alcohols.djvu (142kB)
This file has been downloaded 1735 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
RE: Chlorination of non benzylic aromatic alcohols
Chlorination of the amino alcohol (Phenylalaninol)
C6H5-CH2-CH(NH2)-CH2OH will result in a compound (C6H5-CH2-CH(NH2)-CH2Cl), so my question is what is it called as I need to find its properties ,
solubility ...etc. , I tried 1-pheynyl-2-amino-3-chloropropane, and chlorophenylalaninol, but no success,.....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Chlorination of benzyl alcohols and others.....
Silica Chloride (SiO2-Cl), a New Heterogeneous Reagent, for the Selective and Efficient
Conversion of Benzylic Alcohols to Their Corresponding Chlorides and Iodides
Habib Firouzabadi,* Naser Iranpoor,* Babak Karimi, and Hassan Hazarkhani
SYNTHETIC COMMUNICATIONS
Vol. 33, No. 21, pp. 3671–3677, 2003
pdf
ABSTRACT
Structurally different benzylic alcohols were efficiently converted to
their corresponding chlorides by silica chloride (SiO2-Cl) in CHCl3 at
room temperature. Silica chloride is also able to convert benzylic
alcohols to their iodides in the presence of NaI in a mixture of
CH3CN/CHCl3 in excellent yields.
Key Words: Silica chloride; Benzylic alcohols; Benzylic chlorides;
Benzylic iodides.
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Conversion of Alcohols to Alkyl Halides
Immobilization of HX: [Hmim]X as Halogenating Agent, Recyclable Catalyst and Medium for Conversion of Alcohols to Alkyl Halides
WU, Hai-Hong( ) SUN, Jing( ) YANG, Fan( ) TANG, Jie*( ) HE, Ming-Yuan( )
Chinese Journal of Chemistry, 2004, 22, 619 621
Direct Link URL: http://home.ripway.com/2005-1/247174/ConversionofAlcoholstoA...
Alternate URL: http://host.picturewizard.com/2005-1/247174/ConversionofAlco...
Abstract
HX is immobilized by reaction of halogen acid with methylimidazole, and the formed ionic liquid [Hmim] was used as halogenating agent, catalyst as
well as medium for conversion of alcohols to alkyl halides. Excellent yields were obtained. The halides produced could be easily separated from the
reaction mixture via simple decantation, and the ionic liquid [Hmim]X could be regenerated conveniently by adding equivalent of halogen acids followed
by removal of water.
Keywords halogenation, ionic liquid, alcohol, alkyl halide
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
This article has an interesting section in route to the premise goal, regarding primary aliphatic alcohols, where they get chlorinated easily , hence
since primary alcohols are much harder to chlorinate, both secondary benzylic type alcohol and tertiary alcohols will also
chlorinate..........it's worth a try since its so OTC.......solo
------------------------------------------------------------------------
The Condensation of Aliphatic Alcohols with Aromatic Hydrocarbons. I. The Preparation of Mesitylene and sym-Triethylbenzene
James F. Norris and John N. Ingraham
journal of the American Chemical Society (1938),Vol. 60, No. 6:,pp 1421 - 1423;
DOI: 10.1021/ja01273a04
http://rapidshare.de/files/4435062/The_Condensation.pdf.html
Excerp
.....alcohols react with aluminum chloride and form compounds having the formula
ROAlC12, which decompose when heated and produce RCl + AlOCI.
Note: thanks to Sandmayer for acquiring the citations
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
I think however this article may have some answers to the chlorination problem.... as soon as I can retrive it........solo
Preparative synthesis of primary and secondary alkyl chlorides from alcohols[
N. P. Volynskii1, L. I. Perepelitchenko1 and L. A. Zegel''man1
Russian Chemical Bulletin (Historical Archive),(1987), Volume 36, Number 11, pg.2326 - 2328
ISSN: 1066-5285 (Paper) 1573-9171 (Online)
DOI: 10.1007/BF00957308
http://rapidshare.de/files/10597544/PREPARATIVE_SYNTHESIS_OF...
Conclusions
We have developed a preparative single-stage tosylate method for obtaining chlorides from secondary and primary alcohols which isomerize when
chlorinated by other methods.
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 11, pp. 2506–2508,
November, 1987
Note: was able to secure the article with the assistance of ayush
[Edited on 7-1-2006 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Here is a chapter from
Comprehensive Organic Transformations: A guide to Funtional group preparations
R.C.Larock
on the subject of Halogenation of Alcohols ...all references.............solo
http://rapidshare.de/files/10907799/halogenation_of_alcohols...
[Edited on 12-1-2006 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
(Chloro-phenylthio-methylene)dimethylammonium chloride
(CPMA) an e?cient reagent for selective chlorination and
bromination of primary alcohols
Laurent Gomez,a FrancË oise Gellibert,b Alain Wagnera,* and Charles Mioskowskia,*
http://rapidshare.de/files/10911105/_chlorination_and_bromin...
Abstract
(Chloro-phenylthio-methylene)dimethylammonium chloride reacts smoothly with a variety of alcohols, to a?ord the corresponding alkyl chloride in good
yields. In the presence of tetrabuthylammonium bromide the corresponding bromide is obtained. Selective halogenation of primary hydroxyl groups in the
presence of an unprotected secondary one is described. The mild reaction conditions involved are compatible with the major alcohol protecting groups
as well as with acid sensitive functions like epoxides.
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Vitus_Verdegast
Hazard to Others
  
Posts: 297
Registered: 5-12-2004
Location: Ottoman Empire
Member Is Offline
Mood: tea time
|
|
TCT and TCCA
For the sake of the search engine:
| Quote: | | Wow, that's a real good finding, especially as TCT (TCCA) is OTC available. If they don't already know it, you should post this on the hive, when
they're online again |
No, TCT is not the same as TCCA. It seems that both have been confused with each other for some time.
TCT = 2,4,6-trichloro-[1,3,5]-triazine or cyanuric chloride
TCCA = 1,3,5-Trichloro-S-triazine-2,4,6-trione or trichloroisocyanuric acid
TCT:
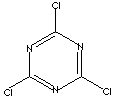
TCCA:
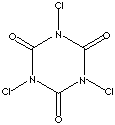
TCT, although not readily available OTC, should be easily and cheaply obtainable from a chemical supply house.
|
|
|
siva
Harmless

Posts: 9
Registered: 13-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by solo
Immobilization of HX: [Hmim]X as Halogenating Agent, Recyclable Catalyst and Medium for Conversion of Alcohols to Alkyl Halides
WU, Hai-Hong( ) SUN, Jing( ) YANG, Fan( ) TANG, Jie*( ) HE, Ming-Yuan( )
Chinese Journal of Chemistry, 2004, 22, 619 621
Direct Link URL: http://home.ripway.com/2005-1/247174/ConversionofAlcoholstoA...
Alternate URL: http://host.picturewizard.com/2005-1/247174/ConversionofAlco...
Abstract
HX is immobilized by reaction of halogen acid with methylimidazole, and the formed ionic liquid [Hmim] was used as halogenating agent, catalyst as
well as medium for conversion of alcohols to alkyl halides. Excellent yields were obtained. The halides produced could be easily separated from the
reaction mixture via simple decantation, and the ionic liquid [Hmim]X could be regenerated conveniently by adding equivalent of halogen acids followed
by removal of water.
Keywords halogenation, ionic liquid, alcohol, alkyl halide |
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Try the alternate URL siva......it works........solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Cyanuric chloride (CC) is a cheap, unrestricted industrial chemical that is a less well known but versatile and efficient chlorinating agent for
carboxylic acids and also alcohols.
If you go to the Acros website and do a catalog search on this compound you will obtain the appropriate literature references.
CC is the cyclic trimer of cyanogen chloride and can be regarded as the acid chloride of cyanuric acid. It is an easily handled solid with a pungent
irritating odor. Its main industrial applications are in the production of various s-triazine agrochemicals and pesticides.
Along with oxalyl chloride, CC has the potential to take the place of the now "politically incorrect" thinyl chloride, ohosphorus trichloride and
phosphorus oxychloride as laboratory chlorinating agents of choice.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
| Quote: | Originally posted by Sauron
Along with oxalyl chloride, CC has the potential to take the place of the now "politically incorrect" thionyl chloride, phohosphorus trichloride and
phosphorus oxychloride as laboratory chlorinating agents of choice. |
Aside from the journal citation in the reference*, do you have any knowledge or experience if the CC will chlorinate a primary(non-benzylic)
aliphatic amino alcohol.....
Note:
I think CC was discussed at the Hive by Aurelius and reviewed by Lego...need to check.....solo
-----------------------------------------------------------------------------------
Cyanuric Chloride. A Novel Laboratory Hydrochlorinating Reagent for Alcohols
STANLERY. SANDLER
J. Org. Chem., 35,3967 (1970)
Attachment: Cyanuric chloride- a novel laboratory hydrochlorinating reagent for alcohols.pdf (243kB)
This file has been downloaded 1603 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Yep, that's the CC route to alkyl halides I had in mind. Note that it also represents a cheap and facile method of generating chloromethane (methyl
chloride) gas from methanol as an alternative to having to buy a lecture bottle or a cylinder.
While the JOC Sandler article does not specifically address aminoalcohols, the Tet.Lett. piece that was up on the Hive did illustrate CC clorination
of the carboxylic acid function of amino acids and peptides without any attack on the amine functionality. So it would appear to have the selectivity
you are looking for. Good luck and good chemistry.
|
|
|
| Pages:
1
2 |