| Pages:
1
2
3 |
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Yes, now I also realised that 70% H2O2 may be the right concentration for monopropellant rockets, but totally unnecessary for the vehicle you want to
build. Just experiment with 30% H2O2 (I have a feeling that the engine won't be easy to build- you need a narrow nozzle).
About the silver powder I don't know, but milling a metal is difficult.
Potassium permanganate is also a good catalyst, much better than MnO2, but when I used it to catalyse the decomposistion of H2O2, the violet colour
disappeared.
I think some colourless manganese salts form, this also needs research.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I would consider a reaction that take place inside a huge syringe. The plunger is pulled outward, lets say,15cm. Some gear system transform this 15cm
in 25ft. That is about 50 times the original movement. You could get a rather precise stopping.
The plunger could pull a string winded (wound? sp? my english fails...) around a 4mm shaft coaxial to a 200mm wheel.
Can you built the car or its given to you?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
The reaction of potassium permanganate and hydrogen peroxide is not catalytic. It is redox, the permanganate oxidizes the perxide to oxygen and water
and the permanganate is reduced to manganese dioxide.
I tried this a while ago, if a solution of potassium permanganate was poured into DILUTE ( 35% peroxide causes the entire contents of beaker to
eject ) peroxide quickly, there is gas given off and a brown precipitate is
produced. I believe the ppt is MnO2 because it still has the ability to decompose peroxide. Hence why the permanganate solution must be poured into
the peroxide quickly, otherwise the formed MnO2 will decompose the peroxide before it has a chance to react with the permanganate. ) peroxide quickly, there is gas given off and a brown precipitate is
produced. I believe the ppt is MnO2 because it still has the ability to decompose peroxide. Hence why the permanganate solution must be poured into
the peroxide quickly, otherwise the formed MnO2 will decompose the peroxide before it has a chance to react with the permanganate.
[Edited on 11-11-2004 by rogue chemist]
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Ok I have some new information if anyone cares. I guess were not allowed to use a fuel cell or an electric cell. The guy in my group came up with this
Idea that we should use some type of teeter totter thingy. This got me thinking if I could build a really small version of an old handcar maybe that
might work. I'm attaching an image incase you don’t know what a hand car is. Anyway, my idea is to hook up a pressurized chamber to a hydraulic
of some type with a hose. This hydraulic would need to do a specific task. The hydraulic will pressurize, that will make it extend when it’s fully
extended it will depressurize and contract to its contracted position. Once it’s in a contracted position, it will pressurize and extend again. This
will give a reciprocating motion. I'm thinking that this type of pneumatic cylinder is a lot like the one in a fully automatic paintball gun. If
any of you think that this type of pneumatic cylinder sounds familiar let me know, I need to get one. All I would have to do is stick this
reciprocating hydraulic under one handle of the railcar. This would mimic the man pumping the handle. Any advice would be greatly appreciated.
[Edited on 12-11-2004 by tom haggen]
[Edited on 12-11-2004 by tom haggen]
N/A
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Edit: Image of hand car.

N/A
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Check the picture.
1- Inject water and lock that syringe.
2- Reaction push out the plunger of larger syringe, pulling the string.
3- String unrolls from shaft, moving the main wheel.
4-Plunger is stopped at the end of its course after having moved your cart 25 feet.
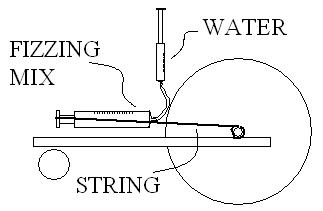
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Dude that seem like you would need a really large syringe or a very violent reaction. Though I really like the simplicity of the invention. I just
remembered this morning apon waking up. When I took a crash corse electronics class at community college they had these automatic pnuematic cylinders
hooked up to a relay ciruit and a source of pressure. I could use that exact same circuit to power my hand car idea, using my chemical reaction for my
soucre of pressure. It seems overly complicated and probably is, and If it doesn't work I might revert to your invention tacho, because it makes
a lot of sense. Don't know if it will go a full 25 feet.
N/A
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tom haggen
Dude that seem like you would need a really large syringe or a very violent reaction. |
I have a 60ml disposable syringe that looks perfect. I bought it in a veterinarian supply shop. Two of them connected like this...
......................water goes here, a T connection
..................................|
..................................V
.............________..||..________
|=====________===________=====|
...would give you about a foot of displacement.
Anyway, I have used a PVC pipe as a large syringe using a piece of rubber as the plunger top and a piece of thinner PVC pipe as the plunger itself. I
used it as a pressure filtering system.
| Quote: |
Don't know if it will go a full 25 feet. |
It will go any distance, it just depend on the plunger displacement and the wheel/shaft diameter ratio. If the ratio is too big, you will need more
energy from the reaction to make it move, of course.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
I think our group is going to try using your method tacho. Mine is way to over complicated. I think we are going to modify the outside diameter of our
wheel in order to make the proper ratio for achieving the full 25 feet. Thanks for your help.
N/A
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Let me suggest that you make a small prototype first.
I have some experience in developing engineering projects, and small prototypes have saved me a lot of trouble before.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
what do you mean make a small prototype? My class project is going to fit on a 6 inch plastic car. If that isn't a small prototype than I
don't know what is.
N/A
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Sheesh!
Does it have to be that small? Those large syringes and main wheel just won't fit there.
Guess we're back to drawing board...
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
No I've figured out a good wheel to axle ratio. By the way where the hell did you come up with this idea? It's freakin brilliant. I just
hope it's something my professor hasn't seen before, though I doubt it would matter.
N/A
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Thanks tom, I also think the idea is good, that's why I insisted on it. I hope it works. Please, post your results.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, Tacho, I think the wound string drive link with inherent limit is very clever.
I think I'm going to stop making comments about situations "going south." By the way is that expression reversed where you live?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Man, I just realized something. How are you suppose to get the liquid to flow down from the top syringe to the bottom syringe filled part way with
baking soda? If you have the top syringe sealed off it wont allow fluid flow through the tube down to the lower syringe with the baking soda. Further
more, if the fluid was able to make it down to the baking soda once the reaction pressurized it would send the fluid back up the hose preventing it
from reacting further. Any solutions to this apparent design flaw?
N/A
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
1- To start the reaction, I would leave some ammount of air along with the baking soda (or other fizzer), about 2 cc should be enough. This air will
be compressed by incoming acid, allowing it to enter the main syringe.
2- After injecting the acid, the smaller syringe is then locked somehow. Insert it in a “restraining slot” carved in the cart, or drill a hole
through its barrel and plunger (at the top) and insert a nail in it. The hole has to be drilled with the plunger pushed all way down, so the holes
align when its content are fully injected.
Hole.......Hole
..|............|
..V...........V
.............________
|=====________=
Some other other possible flaws:
1- The hoses will escape due to pressure, and squeeze the fizzing sludge all over your teacher’s face. Not too bad, but may lower your grade. Make
sure the hoses connections can withstand the pressure.
2- Your cart won’t stop at the end of 25 ft because the larger wheel has gained inertia and will stretch the string and compress the larger syringe,
starting an oscillating movement. May not happen, just experiment will tell...
3- After your cart start moving, I believe it will keep moving, but starting it may be a problem. Again, only experiment will tell...
Edit: Magpie,
We don't have that expression, but you reminded me of a world map printed in Australia, where South was on top and north was at the bottom, but
all the text was properly printed, so Australia was in the top and in the middle of the world! It's all a matter of references...
[Edited on 17-11-2004 by Tacho]
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Apon dreaming about how shitty I might have done on my math test I came up with a much simpler method of injecting the acid. Dont even use a plunger
on the top syringe. just get everything setup, and start pouring the acid in to the top syringe with out a plunger. Once the acid has met the base in
the lower syringe and the reaction has commenced, cap the top syringe with a pressure tight seal. Pressure will begin building up and have no place to
go, unless the lower syringe starts exdending. Im hoping when that happens it will pull more acid down from the top syringe, but experiment will give
results. I'm not to worried about the whole inertia thing yet. I have to get it to move in the first place.
N/A
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
Well, you've lured me out of hiding. I should be doing important stuff for school, but instead... Anyhow, for a quick, practical
solution I think you're on the right track. However, here are some other ideas for moving a device using a wholly contained chemical reaction.
They are of varying applicability.
If one were to line a cylindrical can with a dense material, and then fill it half full with less dense solvent, the changing center of gravity as the
liner dissolved should cause the thing to roll (the motive force is small, though). Variations are easy to come up with. If you want more power (who
doesn't), have the device generate gas on the submerged portions. An inverse waterwheel inside the container would then provide the needed
torque. You'd need a way to control pressure buildup. For greater simplicity, I'm pretty sure a properly constructed rubber cylinder half
filled with hot water will roll, too.
Which brings us to the concept of using heat sensitive materials, rather than gas production. In particular, shape memory alloys provide a great way
to turn heat into just about any mechanical phenomenon you want. If you have a supply of SMA, it could well outperform the piston approach. But
It's gimmicky.
Back to using gas production, how about a reverse peristalic pump? Wrap a soft tube several times around a wheel (so the weight of the vehicle cuts of
gas flow where the tube is squeezed between the wheel and the floor), and attach one end to a gas source, the other to a sink. In order for gas to
flow from the source to the sink, the wheel must roll.
And then, of course, there's the "Cochlea". Take a soft tube and back it with a thin strip of steel (or whatever), forming a spring
that will tend to roll up into a spiral. Starting out in a compact spiral, gas is produced, causing the tube to inflate and the device to uncurl to
its full 25 foot length. So far you've only gotten the front part of the device to the finish line, but you've cleverly designed the
reaction so as the gas is slowly absorbed, at which point the spring action causes it to roll up again, tail first. Of course, you could do a
similar thing using SMA or even a bimetallic strip.
If it was my project, assuming the grade didn't depend on absolute success, I'd go with the Cochlea for the sake of novelty and elegance.
Mechanicly stopped piston schemes are the route of least resistance, though.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
The grade is not really about success, it's more about how well you do on your write up describing your processes, and calculations for designing
your project. Of course, the thing has to at least work, but if it lands on 25 feet or not it not a big deal. I like your intresting suggestions
geomancer. However, I have already decided to use the syringe method and unfortunately I have gone past the point of no return in this dirty whore of
a college term.
[Edited on 17-11-2004 by tom haggen]
N/A
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
tom haggen: I figured as much; for the most part I posted my ideas just because I find them interesting.
Tacho: I have a map like that, but it's cut through the Pacific (I assume the one you mention cuts the Atlantic?) so that Australia is in the
upper left, rather than the center. It's called the "What's Up: South!" map, apparently distributed by ODT, Inc (www.odt.org). I have a laminated copy on my wall, and it's a decent world map, except for the fact that there's some annoying
information about various map projections on the bottom.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Well thanks to all that helped with the tips. Special thanks to tacho whos idea I ended up building off of. Here is the picture of my pressure cart.
Solely built by my hands because the members in my group are mechanically retarted.
I've gotten the car to go a good ten feet. Tommorrow Im going to increase the diameter of the drive wheels as much as possible to reach the full
25 feet. The race is tomorrow wish me luck. I'll try to win this one for sciencemadness.
[Edited on 3-12-2004 by tom haggen]

N/A
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Wow! That’s a beauty!
Congratulations! You have a future in engineering.
Tell the other members of your group to switch their major to literature.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Tom that is wonderful! You should win on design and appearance alone. Let us know how this goes and what your propellant materials are. I would
also like to know what other good designs were entered. 
Don't be dismayed by having to do this all yourself. Just remember that those who do, learn.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Wow its been a long time, I dont know why I never reposted the results. My vehicle did not go very far only like 10 feet or so and I think it had to
go 25 feet or something. If I remember correctly the person who won had a car that had a pressure chamber which was pressurized and then the car was
set on the ground. After the car was set on the ground they had a release valve or something that spun a propeller that powered the car all the way to
the finish line and won the race. I have since given up on chemcial engineering haha, my life has no direction now and I dont know what to go back to
college for. On the brighter side, Im in recovery and i have 18 months of sobriety.
Man that was a cool car I had forgotten all about it untill I looked at this post and saw that picture just now.
[Edited on 17-2-2009 by tom haggen]
N/A
|
|
|
| Pages:
1
2
3 |