| Pages:
1
2
3
4
..
12 |
Pyridinium
Hazard to Others
  
Posts: 258
Registered: 18-5-2005
Location: USA
Member Is Offline
Mood: cupric
|
|
| Quote: | Originally posted by Jome
The compound is turned back into (which?) gas and sulfuric acid when hydrolysed? |
HSO4NO (or if you prefer, ONOSO3H) forms NO, NO2, H2SO4, and probably SO2/SO3 when decomposed.
Merck states the decomposition temp. is 73.5 C but curiously, it also says the NOx will form above 50 C (so which is it?)
Water is said to accelerate the decomp., so even if H2SO4 stabilizes it, you could dil. the contaminated H2SO4 with an excess of water, then boil off
the water. The NOx / HNO3 formed would volatilize, leaving the H2SO4.
Another idea, since ONOSO3H can crystallize... maybe cool the H2SO4 down greatly so it crystallizes out, then decant the liquid? I don't know
what temp you'd need.
Sorry if this already was mentioned in the thread. It's getting late and I'm tired.
|
|
|
haydz
Harmless

Posts: 21
Registered: 1-3-2005
Location: New Zealand!
Member Is Offline
Mood: No Mood
|
|
This sounds very interesting, has anyone had any good amount of H2SO4 come from it? Has anyone tried concentrating it?
|
|
|
neo_90
Harmless

Posts: 15
Registered: 28-6-2006
Location: Sweden
Member Is Offline
Mood: Energetic!
|
|
I know that this thread has been dead for over 9 months.. but I have a questions..
the H2SO4 produced this way, can it become pure enough to make HNO3?
and/or will it be pure enough to work as a catalyst when producing nice smelling esters?
\"If we knew what it was we were doing, it would
not be called research, would it?\" -- Albert Einstein
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I don't see why not. But you may have trouble getting it dry enough to be valuable as a dehydration agent.
Tim
|
|
|
neo_90
Harmless

Posts: 15
Registered: 28-6-2006
Location: Sweden
Member Is Offline
Mood: Energetic!
|
|
when you say dry enough, do you mean a higher %?
cant I just boil the water out?
and, is there a way to finde out what precentage the acid is?
to make HNO3 I've read that it has to be 96%..
\"If we knew what it was we were doing, it would
not be called research, would it?\" -- Albert Einstein
|
|
|
enhzflep
Hazard to Others
  
Posts: 217
Registered: 9-4-2006
Member Is Offline
Mood: No Mood
|
|
Yeah, you can _just_ boil the water out. It's just that it takes a lot of energy. The process now used adds SO3 to water/acid mix and can make
anhydrous acid without additional heating (and the associated product loss)
To check out the strength of your acid, reffer to this thread.
http://www.sciencemadness.org/talk/viewthread.php?tid=5817#p...
Nitric may be made with sulphuric of less than 96% concentration. The only thing is that the distillation will need to be run at a higher temp, will
produce acid with some water in it (hence higher temp needed), in addition to these two points, you will have a lower yield as a result of the greater
amount of nitric being decomposed at the higher distillation temp.
That said, I've successfully made nitric from boiled car battery acid + nitrate salts. Though it still wasn't as potent as that produced by the
distillation of comercial 70% with comercial 98% sulphuric.
ps - don't wear too much cotton (t-shirts, jeans etc) 
|
|
|
tupence_hapeny
Hazard to Others
  
Posts: 131
Registered: 25-3-2007
Member Is Offline
Mood: continuing respiration (touch wood)
|
|
Ummm,
I finally found full-text access to the journal article regarding the oxidation of sulfurous acid to sulfuric acid via freezing the sulfurous acid to
-10C (x3 freeze-thaw cycles), which apparently converts sulfurous acid to sulfuric in 100% yield (NB best would be ~40% as this is the saturation
point of sulfurous acid in water).
Unfortunately, I neither read nor write in Japanese (and the article is in Japanese), full text is available here:
http://www.jstage.jst.go.jp/article/nikkashi/2001/2/2001_125...
Now what I would like to know, is:
(1) The procedure - namely, is the dissolved oxygen just the oxygen that is already in the solution or is H2O2 (or other agent) used?
(2) Whether the 40% H2SO4/H20 solution can be added to with more sulfurous acid - which is then oxidized to more H2SO4 - and if so, whether another
round could be done, converting 80% H2SO4 & 40% H2SO3 to 97% H2SO4 & 23% SO3 via the same route?
Ideally I would like someone to post a translation of this article, I know it is a big ask... However - it would be seriously important - veritably
changing forever the ability of amateur chemists to access a range of reactions cheaply and easily. Even if it cannot be further concentrated by that
method, 40% H2SO4 would be easily prepared - boil it down to concentrate it - then freeze the SO3 (~10C) and filter (I would suggest a porcelain frit
funnel). Add 40% H2SO4 solution to the frozen SO3 and all of a sudden have 80-120% (1:1 or 1:2) H2SO4.
This could alter amateur chemistry for ever...
NB A similar procedure is also apparently possible for the oxidation of nitrous acid to nitric
[Edited on 24-4-2007 by tupence_hapeny]
Attachment: Honda, 'Acelleration of Oxidation of Sulfurous Acid by Freezing' (2001) 2 Chem Soc Japan 125.pdf (307kB)
This file has been downloaded 2782 times
We are all the sum of our experiences, and our reactions to the same
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
sorry by up again this thread, but a few things still disturb me..
| Quote: | originally posted by axehandle :
(a) 3S(s) + 2KNO3(s) --> K2S(s) + 2SO2(g) + 2NO(g) ;sulfur + KNO3 reaction
(b) S(s) + O2(g) --> SO2(g) ;combustion inside the chamber
(c) 3NO(g) + 3/2O2(g) --> 3NO2(g) ;spontaneous at NTP
(d) 3NO2(g) + 3SO2(g) --> 3SO3(g) + 3NO ;catalyzed oxidation
(e) SO3(g) + H2O(l) --> H2SO4(aq) ;absorption
|
and ,from the way which axe have made, what happen to K2S? my worry is which some of it can be oxidised to more SO2 and K2O by the saltpeter and
some of this can fall into H2SO4, impurifying it... any ideas?
EDIT: tupence_hapeny , great document.. has anyone tried this?
Bromic: i've seen another great document which you have posted at another thread from catalytic oxidation of SO2(aq) in presence of some MnSO4 ...
what about build a *somewhat* different and more expensive lead chamber?.. replacing the KNO3 by KMnO4 (i'm sure which at least some MnSO4 are
produced) and fire it inside a chamber with temperature and pressure control (to insure full absorpition of the SO2 generated)...
unfortunatelly by this line of thought , the sulfuric acid whcih can be produced will be very impure , expensive and diluted... but is an idea 
[Edited on 20-6-2007 by Aqua_Fortis_100%]
[Edited on 20-6-2007 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
i found a page with a ton of good info on H2SO4 production:
http://www.sulphuric-acid.com/TechManual/LeadChamber/Lead_Ch...
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
i found this reaction interesting:
2 HNO3 + 3 SO2 + 2 H2O ---> 2 NO + 3 H2SO4
or
4 HNO2 + 2 SO2 ---> 2 H2SO4 + 4 NO
it works according to United States Patent 4155989. seems like it would work awful nicely for making so homemade H2SO4. and i also like because you
don't need the big lead (or plastic) chamber for the gases to react. i'll have to look into this some more.
[Edited on 3-11-2007 by 497]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
so this is my idea for H2SO4 production. seems doable, no 500C temps, no big chambers, no KNO3, no sulfates. sorry about the crude picture... can
anyone find something wrong with it?
[Edited on 3-11-2007 by 497]
[Edited on 3-11-2007 by 497]
Attachment: sulfuric acid.ppt (40kB)
This file has been downloaded 2213 times
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Either you're adding HNO3, or you'll run out of oxidation. You'll need an air bubbler in the NOx column at least.
What's wrong with bubbling air through H2SO3? Doesn't work?
Tim
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
as far as i've seen H2SO3 doesn't convert on its own. i think if it did we'd all be making our own H2SO4 
and why would i have to add more HNO3? the reaction works just fine with HNO2 also. in fact that may end up doing the majority of the work. thanks for
the input.
i have a couple of problems with it though. i am skeptical its going to get much gas exchange with simple bubbling, so i was thinking i would use an
aquarium airstone that produces very fine bubbles. but the problem is i'm not sure where i can get one that will hold up in concentrated sulfuric and
nitric... or maybe theres another way to get good exchange...
also i'm not sure what to use as containers. something fairly tall would definitely be better but is has to have a sealed lid, so glass is not an
option. my first thought was PVC but i'm not sure how well that would hold up in those acids. if it can handle them i might even get clear PVC. if all
else fails i could use some SS 316 pipe, i can weld it too... but in very big diameters that shit is expensive!
well i checked ebay... 5 ft of 2 1/2 inch diameter SS 304 going for $60 plus $20 shipping... not too bad i suppose. 304 should work too shouldn't it?
[Edited on 4-11-2007 by 497]
|
|
|
Armistice19
Hazard to Self
 
Posts: 87
Registered: 19-6-2007
Member Is Offline
Mood: Brain sponge activated!
|
|
Correct me if I'm wrong, but wouldn't bubbling pure oxygen gas through H2SO3 oxidize the acid into the desired H2SO4? If so, Home Depot sells Brazing
kits for 50$. This includes an oxygen, and mapp gas adapter, that mixes the gases into one general outlet via the seperate tubes. Obtaining pure
oxygen is simply a matter of seperating the tubes.
Just a thought,
Armistice.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
no i don't know for sure that straight O2 won't oxidize H2SO3. but it doesnt quite make sence since this entire thread is based on producing H2SO4 and
nobody ever mentioned bubbling O2 through it. well actually tupence_hapeny did talk about in the above post. the fact that honda is freezing and
thawing it 3 times to oxidize it kinda gives me the idea it quite that easy.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Ok, I'll run you through your equations:
Burner: S + O2 = SO2(g).
The SO2 is pumped into a water tank, along with residual O2 and inert gasses N2, CO2, Ar, etc. diluting it.
The SO2 dissolves, and reacts with HNOx from the following step:
SO2(aq) + 2HNO3 = H2SO4 + 2NO2(aq/g) (or hydrogen as units of H2O)
H2SO3(aq) + NO2(aq) = H2SO4 + NO(g)
SO2(aq) + 2HNO2 = H2SO4 + 2NO(g)
The NO gas is passed off into water, where it presumably forms HNO and HNO2. No HNO3 is formed, because no NO2 is present. (If there is, as a result
of circulation it will soon be reduced to NO.)
If NO also has enough oxidizing power to produce sulfuric acid, then you will continually lose N2 gas in the process.
If oxygen is admitted either to the nitrate solution or to the NO gas, you might be able to sustain something here.
Note that the nitrate bath is going to be very rich in water, and you need to add it to the sulfate bath in proportion to the amount of oxidation
required at any given moment in time. Your "sulfuric acid" will very quickly become little more than mere acid rain. One solution would be to aerate
the NO gas and bubble NO2 back into the solution, but this is silly, because to add air, you must remove the inert gasses! You would need pure oxygen
to maintain such a catalytic cycle.
Armistice: are you referring to those 1 pound propane bottle sized oxygen cylinders? The ones that give you eight, count them eight minutes of burn
time? And cost about a dollar per minute of use? Ouch.
Tim
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
why couldn't you supplement in some air in the SO2 feed? wouldn't the O2 pass through the sulfur column and oxidise the NO on its way to the nitrate
column? from what i've read NO is rather quickly oxidized. and i was thinking the water input would be slow, hopefully resulting in fairly
concentrated acid.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
revised version
so i decided a batch process would be much more effective. coments?
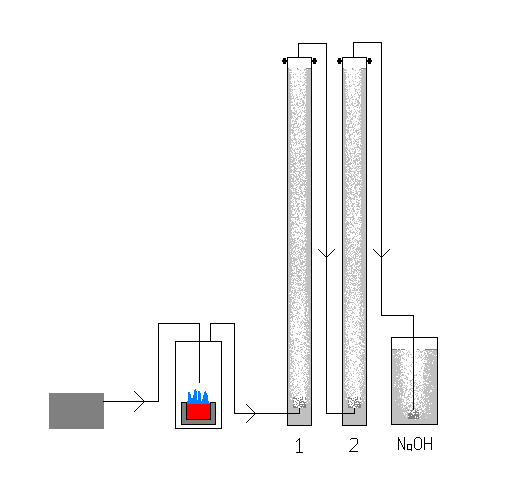
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
and here's the info on it
Attachment: notes.txt (1kB)
This file has been downloaded 2092 times
|
|
|
Armistice19
Hazard to Self
 
Posts: 87
Registered: 19-6-2007
Member Is Offline
Mood: Brain sponge activated!
|
|
I was actually expecting an answer like this one. I was attempting to add a bit of speculation to an old oxidation theory which I combined with your
previous statement about bubbling air through sulfurous acid. I was taking a risk, but now I honestly believe it was worth the informative response. I
also found that my method would actually prove quite practical, but only in the presence of a Vanadium Oxide catalyst, furthermore using that method
would not be categorized as the lead chamber process at all, it’s the contact process. I despise the lead chamber process. I would much rather roast
pyrite from a local “Treasures of the earth” store. Some of you are probably thinking “What? The $10 oxygen cylinders and now this?!?!” well I
might as well end your confusion by stating the fact that never buy my equipment or supplies, I find that chemistry in general is too expensive for my
taste, but that never stopped me from doing it.
|
|
|
bilcksneatff
Hazard to Self
 
Posts: 54
Registered: 11-11-2007
Location: Maryland, USA
Member Is Offline
Mood: Sulfuric
|
|
| Quote: | Originally posted by 497
so i decided a batch process would be much more effective. coments? |
That looks like a very good idea, but I've heard that there's a better way to produce SO2. SO2 is used as a preservative in winemaking, and it is
produced by putting tablets of potassium metabisulfite (or sodium metabisulfite) into the wine. You could find these in any winemaking kits. Haven't
tried it myself, though.
[Edited on 12-11-2007 by bilcksneatff]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Not rocket science
Attachment: jce_7_1138_1930.pdf (116kB)
This file has been downloaded 2406 times
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
Another entirely unrelated question: Could ammonium nitrate be substituted for potassium or sodium nitrate?
Furthermore, a mixture of pottasium nitrate and sulfur would be the same as for gun powder correct? Essentially i would be heating gun powder until it
burns?
Chemkid
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by 497
coments? |
Unless you make the tube going into the first absorbtion collumn longer and higher than the water level it will just flow into the sulfur burner.
How do you plan to keep the sulfur alight? Some form of wick would be beneficial.
|
|
|
bilcksneatff
Hazard to Self
 
Posts: 54
Registered: 11-11-2007
Location: Maryland, USA
Member Is Offline
Mood: Sulfuric
|
|
| Quote: | Originally posted by chemkid
Another entirely unrelated question: Could ammonium nitrate be substituted for potassium or sodium nitrate?
Furthermore, a mixture of pottasium nitrate and sulfur would be the same as for gun powder correct? Essentially i would be heating gun powder until it
burns?
Chemkid |
Ammonium nitrate could probably be used, but there is an easy way to convert it to sodium nitrate. You mix a 2:1 molar ratio of NH4NO3 with sodium
carbonate (Na2CO3) and place it over low heat (actually, I put it next to my woodstove). One mole of NH4NO3 is 160 grams, and one mole of Na2CO3 is
105 grams.
2NH4NO3 + Na2CO3 --> 2NaNO3 + NH3 + H2O + CO2
The KNO3/S mixture is not really gunpowder. Gunpowder is a mixture of KNO3, sulfur, and charcoal all ground together.
[Edited on 13-11-2007 by bilcksneatff]
|
|
|
| Pages:
1
2
3
4
..
12 |