| Pages:
1
2
3
4
5
6
..
12 |
microcosmicus
Hazard to Others
  
Posts: 287
Registered: 31-12-2007
Member Is Offline
Mood: spin up
|
|
Dissolving SO2 in water is easy --- you just mix the gas with water and it dissolves.
Like most gases, it dissolves better at low temperature. More specifically, at room
pressure, the solubility in g/L at different temperatures is as follows:
0C 220
10C 150
20C 110
30C 80
40C 65
50C 50
60C 40
70C 35
80C 34
Your idea of burning sulphur in a container with water, then closing it sounds like
how I proceed. Once the sulphur is burnt and the container closed, I then shake it
around to get the SO2 well mixed with the H2O. As the table shows, you will
get more gas into solution if you use cold water.
The hard part is getting it to disproportionate. I have also heard about the sealed
tube at 150C, however the question is at what rate this reaction proceeds. I once
tried it, heating the tube for an hour but didn't notice any appreciable change.
However, the metal bottle I used was hardly the best vessel for the purpose,
it is just what happened to be at hand. One day, when I buy or make a glass
ampoule, I plan to seal some SO2 solution in it and try again. I suspect that
it will take a long time. Even if it not a practical way of making H2SO4, it would
be interesting to see this reaction happen.
As for solubility of sulphur, since the sulphuric acid is going to be quite dilute,
it should precipitate out just fine, so separation would be a matter of filtering.
Unreacted SO3 could be eliminated by heating, sulphuric acid having a
rather high boiling point.
[Edited on 16-2-2008 by microcosmicus]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Don't even need to heat it. Fill a gas tube with SO2 gas, put it in sunlight or other bright light. Ever so often look through the tube lengthwise
or as near as you can get, it will become foggy.
3 SO2 <=> 2 SO3 + S
A problem with working with gases is the low density. Three moles of SO2 is going to occupy about 67 liters at STP. This would yield one mole of
sulfur, roughly 16 cc, and two moles of SO3, between 50 and 90 cc depending if solid or liquid and which form, or about 110 ml of H2SO4. Now it's
true that you start out with only about 55 cc of sulfur to make the SO2, it's just the the volume of gas is inconvenient.
I can envision a flask, strongly illuminated , with a fractionating column - lots of surface area, both held at about 100 C. As sulfur forms it
drifts to the surfaces, collects, and molten runs down to the bottom of the flask. The SO2 and SO3 pass through the column, the SO3 is condensed in
another flask at say 20 C. The system is sealed, unreacted SO2 just drifts about until it reacts. It could be pressurised, which should help due to
the 3:2 volume ration.
Some SOx will dissolve in the sulfur, some SO2 will dissolve in the SO3, how much I don't know.
It's a very slow process, you would want a concentrated source of SO2, say liquefied from a sulfur burner and kept under pressure, and a way to feed
it into the system whenever the pressure dropped low enough. It's not very practical, more of a intellectual joyride.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Could you bypass the issues of large volumes by liquefying the SO2? It boils at -10C or 34 PSI at room temp. Hopefully it can actually decompose in
liquid phase. It would need to be contained in glass to decompose it with light. Shouldn't be a problem in large borosilicate tubing or the like. That
would be pretty awesome if you could just set a tube of liquid SO2 out in the sun (or 150 watt metal halide that I happen to have  ) for a few hours/days/weeks and come back a have a tube full of SO3. Maybe even use
a little parabolic reflector to speed things up. Somehow I doubt it would be that easy. ) for a few hours/days/weeks and come back a have a tube full of SO3. Maybe even use
a little parabolic reflector to speed things up. Somehow I doubt it would be that easy.
Also I've found that 32%H2SO4 + 50%H2O2 reacting with copper metal produces quite a lot of nice clean SO2 (maybe some O2 too) and quite a bit of heat,
that tends to start to boil it after a while.
Edit: I found pressure specs for borosilicate tubing, 38 mm dia 4 mm wall can handle 200 psi, so there should be no problems with pressure as long as
the end caps can be affixed strongly. Having a means of getting the liquid in and out that is resistant to SO3 might be a challenge though. Or you
could just not worry about it and break the tube open when you get the SO3 out.
[Edited on 16-2-2008 by 497]
|
|
|
Pixicious
Harmless

Posts: 31
Registered: 28-1-2008
Member Is Offline
Mood: Smiley
|
|
I put together a fosters beer can and make an attempt.
I have something with a ph 2 and something which turns limus paper red.
What tests are there to determine whether what I have is H2SO3 or H2SO4?
I can see myself having either H2SO4 or H2SO3 because from reading it is said that SO3 in small amounts is given off and is the smoke you see and the
ph is higher than I would expect for H2SO3.
I have a flask which can be made air-tight, do I fill this with hydrogen before sealing or do I keep it with normal air before heating?
I'n going to give that a go this evening.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I'm pretty sure the pH of real H2SO4 is going to be quite a bit lower. Dip a paper towel in it, it will start to dissolve if its H2SO4 of any decent
concentration, I'm pretty sure it won't dissolve in H2SO3. How much water did you add? The beer can is only going to hold like 0.01 moles of SO2, with
very much water its going to be very diluted.
From what I've read its not that easy to convert H2SO3 > H2SO4 without some more extensive manipulation.
I have no idea what you're saying about the hydrogen...
|
|
|
Pixicious
Harmless

Posts: 31
Registered: 28-1-2008
Member Is Offline
Mood: Smiley
|
|
About 20ml of water was put at the bottom of the can and the experiment has been run 4-5 times to increase the concentration. From 1-10g of sulphur
have been burn with an unknown percentage escaping before the cap was put on.
I wish I had done it with a little more accuracy.
I will try the paper towel test right now.
|
|
|
Pixicious
Harmless

Posts: 31
Registered: 28-1-2008
Member Is Offline
Mood: Smiley
|
|
The paper towel was placed inside for 10-15 seconds and hadn't dissolved. I'm worried I may have lost a little since the ph is now reading at about
3..
The only reason I spoke about the hydrogen is due to a reaction with the air when heating inside a sealed container.
I will nake a couple batches of liquid in this way and put to one side until a) they all have a ph of 2 and I'm satified I have enough before I can
make an half decent attempt at making sulphuric acid in this way.
I'm wondering if I can buy a 2l coke bottle and burn more sulphur to increase the production rate. I will also be able 'to see' when the flame dies
and all the SO2 and SO3 has been dissolved,
[Edited on 18-2-2008 by Pixicious]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Hey, anyone got any I2 laying around?  I just realized that you could use part of
the iodine-sulfur process to make good concentrated H2SO4. I just realized that you could use part of
the iodine-sulfur process to make good concentrated H2SO4.
I2 + SO2 + 2 H2O -> 2HI + H2SO4
Then recycle the iodine:
2HI -> H2 + I2
I also found out that liquid sulfur reacts with SO3 to make SO2... not good. Hopefully its not the case for solid sulfur.
[Edited on 17-2-2008 by 497]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by 497
Hey, anyone got any I2 laying around?  I just realized that you could use part of
the iodine-sulfur process to make good concentrated H2SO4. I just realized that you could use part of
the iodine-sulfur process to make good concentrated H2SO4.
I2 + SO2 + 2 H2O -> 2HI + H2SO4
Then recycle the iodine:
2HI -> H2 + I2
I also found out that liquid sulfur reacts with SO3 to make SO2... not good. Hopefully its not the case for solid sulfur.
[Edited on 17-2-2008 by 497] |
You're referring to the Bunsen reaction for the first part. It's one way of making aqueous hydrogen iodide in the lab.
The reaction is an equilibrium, too high of a concentration of the acids drives it towards I2 + SO2 + H2O. This includes distilling the HI-H2O
azeotrope away from the mix.
In the IS cycle to produce H2, the separation is accomplished by using an excess of I2 and some starting HI to form two phases, HI-I2-H2O and
H2O-H2SO4. The excess iodine, plus an excess of water, is used to help drive the reaction to HI + H2SO4.
In that case it typically is run at 110 to 130 C, with cooling as it is exothermic.
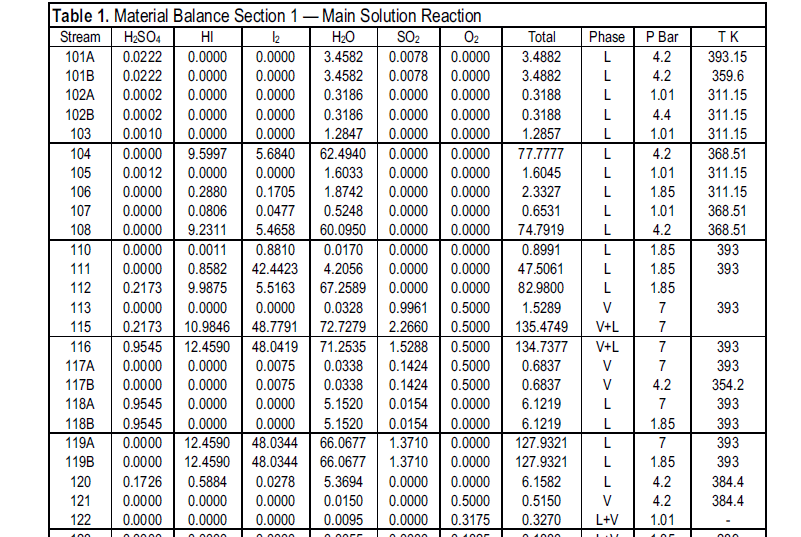
I'm attaching part of a material balance sheet for the Bunsen section of a ISH plant.
section 115 is the feed into the Bunsen reactor, run under a bit of pressure.
116 is the flow out of the reactor into a phase separator
117A is the off gases
118A is the H2SO4 phase
119A is the HI-I2 phase
Note that both acid streams are fairly dilute, the H2SO4 also contains a little SO2 which is no big deal. However the acid concentrations, plus the
large of iodine used, might discourage you; this is especially true in the USA and Oz where the element is contraband.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Yes I just found out about the concentration issues on my own... how frustrating. I suppose that process is pretty much out of the question.
I found an interesting reference in the attached pdf on the "Westinghouse" process that involves electrochemically producing H2SO4 from SO2. I imagine
the concentration would not be high though.
SO2 (g) + 2H2O(aq) 􀃆 H2SO4 (aq) + H2 (g)
Also does anyone know anything about the possibilities of SO2(l) -> SO3(l) + S(s) ? It seems like a good way to solve the problems of large volumes
of SO2 gas...
Attachment: lect5.pdf (2.2MB)
This file has been downloaded 1562 times
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Here's some good info on the Westinghouse cycle. They talk about electrolyzing acid up to 80 or 90 w-%, and they're more worried about efficiency, it
probably could go even higher since electricity costs wouldn't be much of an issue. I'm kind of surprised I've never heard that this could be done.
[Edited on 17-2-2008 by 497]
|
|
|
Pixicious
Harmless

Posts: 31
Registered: 28-1-2008
Member Is Offline
Mood: Smiley
|
|
Then what I currently have (and gathering) in a jar is H2SO3 which when heated will produce H2O + SO2.
I do have some iodine. Could it be worth mixing the iodine and H2SO3 into a sealed container and heat upto 120C? If this is a result the two should be
pretty pure, if the reaction is allowed to complete and correct quantites used.
I will have a method of producing pure (as close as after opening) H2SO4, in maybe a reasonable quanity.
[Edit] I knew I bought that iodine for a reason.
[Edited on 18-2-2008 by Pixicious]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
As I already said, the reaction is reversible - mix H2SO4 and HI and you will get some I2, H2O, and SO2. An excess of I2 and H2O is used to force
the reaction, the concentration of the acids produced is less than 20%. Use less water, more SO2 and I2 will remain unreacted.
|
|
|
Pixicious
Harmless

Posts: 31
Registered: 28-1-2008
Member Is Offline
Mood: Smiley
|
|
I heard you.
What you are saying is I should release the gas at the end of the experiement and boil the excess water forming a concentrated H2SO4.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
That might work, the problem is you will end up with very little. I'd say at most 5 grams from a 2 liter bottle. If I were you I'd try like a plastic
55 gallon drum or a big trash can that you can seal. In theory 55 gallons of SO2 gas could yield around 910 grams H2SO4. I doubt you could get that in
real life, for one thing you're not going to be able to fill the container completely with SO2 by burning it in the container although you could do
better with straight O2 injected.
I think aqueous conversion would be much better, that same 55 gallons of SO2 gas could be dissolved in just 3 liters of cold water. Its been mention
in this thread I think, and it sounds worth trying, freezing the aqueous SO2 to convert it. I think they say 3 freeze-thaw cycles and its completely
converted. I'm not sure how concentrated the SO2 can be though. You'd still have to concentrate it a lot too, you'd only get around 20 or 30%
concentration initially.
What I may do some time when I have the chance is fill a 5 gallon bucket with ice water, build a good sulfur burned and pump straight SO2 into that
water. It should dissolve about 3 kilos of SO2, yielding in theory about 5 kilos of H2SO4. Thats a lot of boiling to concentrate that all... at least
20 kilowatts of energy. And considering I can buy 5 gallons of battery acid (8 kilos H2SO4) for $15, it seems like too much work... unless maybe you
had an enormous sulfur burner that saturated a 55 gallon (makeing 70 kilos H2SO4!) drum of water with SO2... sigh... maybe some day if H2SO4 becomes
regulated.
|
|
|
Pixicious
Harmless

Posts: 31
Registered: 28-1-2008
Member Is Offline
Mood: Smiley
|
|
| Quote: |
...and it sounds worth trying, freezing the aqueous SO2 to convert it.
|
Are you saying freezing the aqu SO2 will convert it to SO3?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Pixicious
I heard you.
What you are saying is I should release the gas at the end of the experiement and boil the excess water forming a concentrated H2SO4.
|
The liquid will be a mixture of water, H2SO4, and HI. Concentrating it will result in the reverse reaction, at least to some extent. So as you start
to boil off water, you'll be driving SO2 and some I2 off.
This is why the sulfur-iodine cycle projects use a major excess of I2 and have some HI in the input stream, the two liquid phases formed allow the
separation of the H2SO4 from most of the HI.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Pixicious, yes you are right it should if done correctly convert SO2 to SO3 which will immediately be converted H2SO4, probably fairly dilute, but at
least it'd be cheap... assuming you can make an effective sulfur burner.
|
|
|
Pixicious
Harmless

Posts: 31
Registered: 28-1-2008
Member Is Offline
Mood: Smiley
|
|
SO2 + 2H2O -> H2SO3 + H2O -> 2H + SO3 + H2O -> H2SO4
I assume then the reaction must be something like that.
I'll give it a try this evening. I won't doubt you I just don't see it.
[Edit] So far so good. I made a new batch of H2SO3 this morning with excess water. It is in the freezer at the moment. I'm assuming an excess of water
is needed for the reaction. It had a ph of 2-3. Limius was slightly red.
There is a solution which is taking longer to freeze than the water. won't try filtering it off until it has been frozen and allowed to thaw three
times. The lid has been and will be sealed throughout. I will seperate the two out on the fourth freeze and see what they both are. It would be
interesting to me to find out why.
497: My suphur burner is comprised of a 2litre coke bottle cut in two with the bottom of a fosters can supported half way by two metal pieces of a
clothes peg. There is a side window and I'm using selotape as a sealent. Works rather well. It is lit and sealed and the sulphur burns for maybe five
minutes (I use a mini blowtorch to light as much sulphur as I can) I can run in batches, after 3-5 a PH of 2 is averaged. The amount of water I use is
about 100ml.
[Edited on 23-2-2008 by Pixicious]
[Edited on 23-2-2008 by Pixicious]
|
|
|
Pixicious
Harmless

Posts: 31
Registered: 28-1-2008
Member Is Offline
Mood: Smiley
|
|
It didn't work I am afraid. Sorry 497 gave several attempts. (my previous post has no edit button)
Would it be possible -at all- to produce SO3 from Na2SO4?
|
|
|
LSD25
Hazard to Others
  
Posts: 239
Registered: 29-11-2007
Member Is Offline
Mood: Psychotic (Who said that? I know you're there...)
|
|
Here is something interesting and funny at the same time:
http://blog.modernmechanix.com/2008/03/05/dangerous-acids-ma...
BTW The trick with the freeze/thaw aqueous variant is to keep it in the freezer for a whole lot longer than they say, until the whole of the solution
is is absolutely solid (took hours in my home freezer) and then defrost the lot (clathrate included - around 15-20C). I am in the process of
defrosting one of my attempts, the liquid in this, admittedly dilute, sample when half-defrosted is at PH.1-2 (PH 1-14 paper). That sort of suggests
it ain't likely to be sulfurous acid, that and the precipitation of calcium sulfate as the conversion took place.
Whhhoooppps, that sure didn't work
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
In aqueous solution, SO2 is oxidised by aerial oxygen.
You could try bubbling air into your sulfurous acid, very slowly, to avoid expelling too much of the SO2 and give the oxygen time to react.
|
|
|
LSD25
Hazard to Others
  
Posts: 239
Registered: 29-11-2007
Member Is Offline
Mood: Psychotic (Who said that? I know you're there...)
|
|
I was actually referring to that Japanese article, where they use dissolved oxygen to oxidise H2SO3 in aqueous solution. They claim 100% conversion
via three 1 hour freeze cycles, I did not find this to be accurate, in fact, overnight freezing - three to four times - was required to change the
properties of the material when allowed to defrost at room temp (the clathrate increased then decreased to nothing on the fourth defrost cycle). I
really should use a hydrometer to work out the spec.grav of the suspected sulfuric acid. It would also have helped to have checked the PH of the H2SO3
mixture on each thaw cycle to see if it decreased or increased during that time.
PS Can I use Calcium chloride instead of barium to ascertain the identity of the acid?
Whhhoooppps, that sure didn't work
|
|
|
Armistice19
Hazard to Self
 
Posts: 87
Registered: 19-6-2007
Member Is Offline
Mood: Brain sponge activated!
|
|
We DON'T need people advocating criminal activity here. Kindly take your "business" elsewhere. This is a CHEMISTRY forum.
Edited by vulture
[Edited on 7-4-2008 by vulture]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: |
Would it be possible -at all- to produce SO3 from Na2SO4 |
https://sciencemadness.org/talk/viewthread.php?tid=10217
|
|
|
| Pages:
1
2
3
4
5
6
..
12 |