Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
Homemade lid for electrolysis cell
ive heard people thinking about melting their own plastic discs, but actually rarely heard people asking about how to make a proper electrolysis cell
lid?
i recall having a thread somewhat about this, but i could not find it
anyways here goes my idea
dumping scrap paper in water followed by tearing it apart gives a uniform paper mass
this can be somewhat dried out followed by mixing with wooden glue, this can then be pressed into a desired shape although it takes abit to master it
and can be quite frustrating to work with
it could be shaped into a cone without tip, as with ground glass to fit onto a jar, holes could be drilled in it for inserting electrodes
although wooden glue is somewhat soluble, it could be coated with glue of any kind or perhaps simple wood? plastic film?!
another idea i had was to make a wooden piece either conically shaped on the edges to fit a potential electrolysis cell, wood should be decently
resistant towards heat, another problem might be that it could end up bending alot when exposed to moisture
when it would happen to get wet, i assume it would plug alot better if it doesnt bend totally out of shape, if an wooden plate was placed ontop of a
jar of fx glass, and a weight was placed there aswell to keep it down with simple gravity, when it would then get wet it should very effectively keep
pressure in the cell, and thus leading the chlorine out from the cell controlled rather than in 360*
yet another idea ive had was to use a machine to make a sort of line in the wood, the wood could be filled with a rubbery material
there are tools to cut lines in wood, i really have no idea what theyre called but a spinning bit with a razorblade on one side removes wood it is
pushed against
the rubbery material could when set to harden be seperated with aluminium foil which could then be removed after hardening, where the shape of the
container could be inserted perhaps with a bit of gravitational assistance to keep it perfectly closed
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I think that would work, I wouldn't do it because I have a good lid already, but you could try it.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
I just use a 5 gallon bucket with a gamma seal lid installed on it.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
It's here.....
http://www.sciencemadness.org/talk/viewthread.php?tid=24097#...
... you Dumb Ass!
|
|
|
phlogiston
International Hazard
    
Posts: 1375
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
You have not said what you are going to electrolyse, but if you are planning to make chlorate/perchlorates, you will want to consider that when a
paper or wood lid becomes impregnated with powerfull oxidisers, it becomes a fire hazard.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
http://site.bayteccontainers.com/image/gamma-seal-lid-parts-...
seems like a brilliant solution for decent sized electrolysis, but yes it was about ClO3, it could potentially become a fire hazard, this is why it
would perhaps be a good idea to cover / coat the wood/glue mass somehow
i guess i couldnt find it was because i kept including electrolysis in the search terms, but i did search alot for it
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I recall making a rocket fuel, that was a mixture of paper, wood glue, and an oxidizer, I think I used KNO3, but KClO3-4 would
likely be even worse(stronger/powerful).
Yes, here's the link:http://markus-bindhammer.blogspot.com/2011/08/newspaper-rock....
[Edited on 5-2-2014 by Zyklonb]
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
very interesting!
it could probably be pushed abit back up the tube or this could be done before rolling it, then a gypsum/sand or even dirt plug could be made to make
it a very easy to make rocket motor
another thing..
when making a cell its often not a good idea to fill it 100% up, making the liquid have contact with the top of the cell, this would make some
problems with getting the gasses out if a simple design would be used..
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
In the video, the rocket seems to burn slower than would be required to get a rocket like that off the ground.
But it's very unlikely that you'll incorporate any KClO3 into the lid, and even more unlikely that you'll accidently light it on fire.
[Edited on 6-2-2014 by Zyklonb]
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
indeed, considering it will stay slightly wet
the water that would get into it i assume would be as condense water, and again a thick layer of paint should do the job
epoxy glue wont do, when heated too much it just turns useless, also it doesnt seem very happy about Cl2 or H2, turns yellow and stops sticking
i think one mistake they might have done was to make the hole all the way through, potentially resulting in a uncontrolled burst, rather than it going
only 33% up then stopping, it could be done using finely divided paper and then wetten it with glue and KClO3
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Why ordinary plastic cover doesnt work? Also, you could install a submerging tube around the electrodes if problematic gases are formed. It will
collect the gases within the liquid gas layer airtight and you just install tube to it and lead the gases safely out. Like this:
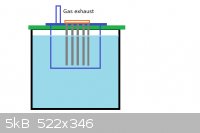
PVC plastic will hold well against all chlorine compounds, including elemental.
Wood will just simply suck all the moisture and rot away sooner or later. You could apply layers of lacquer or paint for it, but at that cost you can
get cheap plastic covers from anywhere, secondly, you can use soldering iron to cut the plastic if you live in a place where power tools are too lousy
to use.
[Edited on 16-2-2014 by testimento]
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
I a larger vessel you are going to want to pump extra air with something like an an aquarium pump. This is just to keep the solution circulating more
so than anything else. If you're worried about the chlorine then just bubble the exhaust through your favorite hydroxide solution to capture the
stray chlorine. If you don't circulate the solution somehow in a larger vessel it will develop temperature variants locking part of the electrolyte
away from your electrodes.
|
|
|
I Like Dots
Hazard to Self
 
Posts: 69
Registered: 10-4-2013
Member Is Offline
Mood: frisky
|
|
Wood glue, paper mache? you are complicating this. I made a hydrolysis cell for hydrogen, and I just used a empty plastic jar with a plastic lid.
3 holes: positive, neutral, gas. seal with whatever, I used hot melt glue.
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
Sheets of Plexiglass (MethylMethaCrylate, speelling probably wrong!!) work ok. Easy to obtain and easy to drill etc.
|
|
|