| Pages:
1
..
3
4
5
6
7
..
13 |
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
2 days ago I used a procedure to make a mix of DNT/TNT posted on an another forum. It uses ammonium nitrate (I used sodium nitrate, 87 g) and H2SO4 (I
used boiled down acid from a old car battery, 125 ml). And I used 20 ml of toluene. I got a red liquid that during washing solidified to a yellow
solid that is a little waxy with some needles in it and a very weak smell.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
The "perfect" method?
I'm looking for the "perfect" method for making TNT. One which doesn't require oleum and where the acid is reused for each precursor without any
regenerating. Or would it be more expensive than using fresh acids for each step? From what I can gather you really don't need more than 65% HNO3
before the last step, and even then you can use it with a reduced yield. True?
I'd like to do a full synth including acid recovery, so it would be beneficial to keep the volumes down to a minimum. Not quite sure if the acid could
be distilled without a dilution, I expect it could still contain some dissolved M/D/TNT that could mess up the process. If the spent acid is strong
enough one could possibly distill 95%HNO3 straight off the spent acid. The alternative would be to dilute, still of 65% and then do an extractive run
to get the good stuff.
Any thoughts on this?
PS: Fashist, AFAIK there is no such thing as dry/anhydrous magnesium nitrate. It is fully hydrated as the hexahydrate, and while it can be dehydrated
to a certain degree it has never been isolated in a completely dry state. I believe the mono or dihydrate is as far as you can go.
Interesting to hear that you've succeeded in this process, my attempt failed completely. Any details you'd care to share with us?
[Edited on 15-2-08 by Fulmen]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
I really can't comment of the concept of "perfect" but the re-use of acid has been discussed and implemented over time and referred to as a one pot
synth on a lab scale. I think you can find a few examples in common literature. I know that Ledgard had one or two examples in his Preparatory Manual
of Explosives; however the citations he lists are famous for being wrong. I looked up some of the patents listed for certain primaries and they just
plain didn't exist.... but the "one pot" synths are listed there, plain as day. I know you can get some of them to give you mixed nitrated materials
with no problem.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Thanks, I'll do a search and see what turns up.
Edit:
Not too much on the net about this, but after calculating a bit it seems to be simple. Each reaction step produces water (one mol per mol toluene),
but not enough to exclude it from further use. Nitric acid is also lost, but this can be added back.
According to this method:
http://www.sciencemadness.org/talk/viewthread.php?tid=29&...
one can use the acid from step 3 and simply add 70% HNO3, and the acid from step 2 can be used for twice the amount of MNT.
This seems quite promising indeed. Still need a distill for the 100% HNO3 and acid regeneration though.
[Edited on 15-2-08 by Fulmen]
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
OK, lets run through the math.
The synthesis calls for the following steps:
1: 10g Tol + 15 ml 50% HNO3 + 30 ml 50% H2SO4
2: 14,7g MNT + 11 ml 70% HNO3 + 20 ml 98% H2SO4
3: 19g DNT + 7,5 ml 98% HNO3 + 22 ml 98% H2SO4
The acid for step 3 contains:
39,7g H2SO4
11g HNO3
1g H2O.
The nitration will consume 1 mol of HNO3 and produce 1mol of water per mol DNT, so the spent acid will contain:
39,7g H2SO4
4,4g HNO3
2,9g H20.
Step 2 calls for:
36g H2SO4
11,2g HNO3
4,8g H20
A bit more sulfuric acid shouldn't change anything as long as the total acid concentration is the same, so by adding 6,6g 65% HNO3 and 2,5g 100% HNO3
the final composition would be:
39,7g H2SO4
11,2g HNO3
5,2g H20.
After the second step the acid will contain:
39,7g H2SO4
4,4g HNO3
7.2g H20
Step 1 calls for:
21g H2SO4
9,75g HNO3
30,8g H2O
So the acid from step 2 is actually more concentrated than required, so adding 65% HNO3 (8,5g) and enough water to prevent oxydation and we're done.
Total consumption should be 39.7g H2SO4 and 23.3g HNO3 for a 23g batch of TNT.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
| Quote: | Originally posted by Fulmen
I'm looking for the "perfect" method for making TNT. |
Then you're probably best off reading COPAE, Sanford, Fedoroff, Urbanski, etc. and doing your own experimentation. Let us know when you've found it.
[Edited on 15-2-2008 by S.C. Wack]
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Perfect is a matter of definition, the perfect industrial process and the perfect amateur method are two very different things. All the "good" cources
like Davis and Urbanski seems to list methods using oleum or large quantities of 100% nitric acid.
The method from Philou seems better for small quantities and limited supplies and as far as I can tell this is a verified method. It still require
100% NA but there might be possible to adapt it to a nitrate-method. Another way would be to make it from calcium nitrate and sulfuric acid. It
doesn't work for pure NA as you just can't separate it from the precipitated calcium sulphate in any resunable yields, but washing it out with more SA
could work.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Perfect is a matter of experimentation, and the perfect amateur method in this case I suspect to be pretty close to that in the references that I
mentioned. Until you have product in hand from internet methods, crapbooks, or patents, you've only got talk.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I won't argue with that, talk is cheap. Unfortunately chems and glassware are not. Hopefully a few (!) hours of reading will save me from a few wasted
experiments. I haven't sifted through all the industrial processes in Urbanski yet, perhaps they don't look so bad after I calculate the yields a bit
better.
But I suspect these are optimized not only for yield and purity but also for time and volume since production capacity is largely a factor of batch
sizes and reaction time.
|
|
|
zeppelin69
Harmless

Posts: 46
Registered: 20-2-2007
Member Is Offline
Mood: No Mood
|
|
I thought I would attach this link for reference. I am Masonjar Chemist on this forum (as well as RS in anyone cares). I created my own procedure for
a one step TNT synthesis and it worked. Pictures were also included, I hope this isn't considered advertising, I'm not looking to break any rules
here.
http://www.dererumomnis.org/bbs/index.php?topic=328.0
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Nice work, Zeppelin. Seems like you've got almost a full yield from your toluene, but it does use a lot of SA (100ml) and AN (82g). A 3-step synth
should be a lot more efficient (for multiple batches that is) and probably easier to perform as well. With a single stage you risk oxidizing the
toluene in the beginning and at the end there may be too much water to get full conversion to TNT.
I haven't desided on the choice of chems yet, but I am looking more and more to AN/SA. Sulphuric acid is more available than NA, and unless one is to
recover the acid it really doesn't matter too much. I would like to do a full synth including recovery, but the glassware i need for that isn't cheap.
[Edited on 18-2-08 by Fulmen]
|
|
|
Bonome
Harmless

Posts: 6
Registered: 19-2-2008
Member Is Offline
Mood: No Mood
|
|
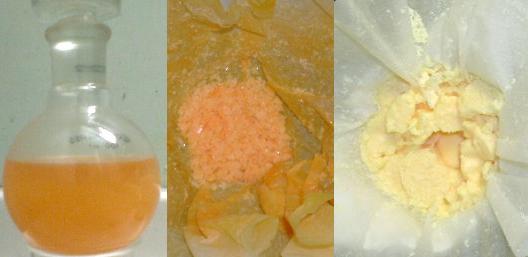
3 pics here, MNT, DNT and finally TNT
My MNT is contamined with DNT that's why it is orange.
[Edited on 20-2-2008 by Bonome]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Very nice Bonome. Could you give an account of your synthesis?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Bonome
Harmless

Posts: 6
Registered: 19-2-2008
Member Is Offline
Mood: No Mood
|
|
Hi chemoleo, Like you can see I used 3 step method.
Here is the details of each reaction.
Sulfuric acid is from Drain opener and has not been concentrated. The concentration is approximately 94%. I based my calcul with that. For each
nitration I used different stochiometrical amount. MNT: 1.1, DNT: 1.2, TNT: 1.5
Some people said to use 1.1 for each nitration. This doesn't work for the last nitration if you have sulfuric acid <98%
My nitric acid was made with ammonium nitrate. I did not distillate the acid because the sulfate doesn't disturb the reaction. The nitrate and
sulfuric acid was mixed and I waited reaction to complete before adding it to the aromatic.
Of course all the nitration was under stirring (Magnetical is my case). I hope you have all the information you wanted.
------------------------------------- Reagents --------------------------------------
Ammonium Nitrate: 0,076 kg
Sulfuric Acid: 0,141 L
Toluene: 0,080 kg
Water: 17 mL
Total Batch Size: ~ 0,286 L
---------------------------------- Temperature ------------------------------------
Starting: 25-30 °C
Finally: 50-55 °C
Final Reaction Time: 1.5 hours
---------------------------------------- Yeild -----------------------------------------
Theoretical yield (%): 97%
Theoretical yield (kg): 0,115 kg
Theoretical yield (L): 0,1 L
--------------------------------------------------------------------------------------------
------------------------------------- Reagents --------------------------------------
Ammonium Nitrate: 0,081 kg
Sulfuric Acid: 0,149 L
M.N.T: 0,115 kg
Water: 5 mL
Total Batch Size: ~ 0,29 L
---------------------------------- Temperature ------------------------------------
Starting: 50-55 °C
Finally: 80-85 °C
Final Reaction Time: 2.0 hours
---------------------------------------- Yeild -----------------------------------------
Theoretical yield (%): 97%
Theoretical yield (kg): 0,148 kg
Theoretical yield (L): 0,112 L
--------------------------------------------------------------------------------------------
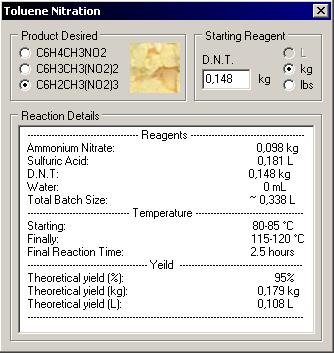
------------------------------------- Reagents --------------------------------------
Ammonium Nitrate: 0,098 kg
Sulfuric Acid: 0,181 L
D.N.T: 0,148 kg
Water: 0 mL
Total Batch Size: ~ 0,338 L
---------------------------------- Temperature ------------------------------------
Starting: 80-85 °C
Finally: 115-120 °C
Final Reaction Time: 2.5 hours
---------------------------------------- Yeild -----------------------------------------
Theoretical yield (%): 95%
Theoretical yield (kg): 0,179 kg
Theoretical yield (L): 0,108 L
--------------------------------------------------------------------------------------------
The quantity was generated by a program I made to calcul all according to my 94% sulfuric acid, here is a screen shot. I create this program for big
quantity so the value are rounded alot for little batch.
[Edited on 19-2-2008 by Bonome]
|
|
|
Bonome
Harmless

Posts: 6
Registered: 19-2-2008
Member Is Offline
Mood: No Mood
|
|
I forget to said that the toluene I used was pure
[Edited on 21-2-2008 by Bonome]

|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Gosh, did you really use 120 liters of toluene? That would make a HUGE amount of TNT!
|
|
|
Bonome
Harmless

Posts: 6
Registered: 19-2-2008
Member Is Offline
Mood: No Mood
|
|
yes there is alot of toluene lolll but I will not have to buy more for alot of time  and no I don't make a reation with 120 Liters... I don't have the appropriate equipement to do that. I can make 12L batch max. and no I don't make a reation with 120 Liters... I don't have the appropriate equipement to do that. I can make 12L batch max.
[Edited on 19-2-2008 by Bonome]
[Edited on 19-2-2008 by Bonome]
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
I Found Interesting Patent
http://www.freepatentsonline.com/WO2005005342.html
Nitric Acid+Trifluoromethanesulfonic acid+DNT--Heat-->High Purity Tnt
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Triflic acid is not particularly cheap (~ 2 EUR/g for reagent grade). It is many, many times more expensive than TNT. Using it to make a nitrating
mixture is not particularly reasonable.
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
Its true.
Merck Trifluoromethanesulfonic acid is 56 eur per liter.
|
|
|
markgollum
Hazard to Self
 
Posts: 53
Registered: 21-2-2004
Member Is Offline
Mood: No Mood
|
|
To Bonome and others who believe they have made TNT:
How do you know that what you have is in fact TNT and not DNT or a mixture of the two?.
You should take the melting range before you tell people what you have.
The color of TNT is heavily dependent on its ph history, even things like a bicarbonate neutralization or a sulphite wash can drastically affect the
color if not done correctly.
TNT melts at 80.1C and I would not tell anyone that a certain procedure made TNT until the unsymmetrical isomers have been removed and the product
melts no lower than about 75C.
DNT impurities in TNT drastically reduce the melting point and cause the product to exude an oily DNT/TNT mix over time. The melting range is very
important and is really the only way to determine the quality of your product.
I really don’t understand how people and even the literature can justify throwing around yields like 97% for non analytical or industrial processes.
I have done a fair bit of chemical syntheses and I am not ashamed to say that I HAVE NEVER GOTTEN a final product with a yield in that range for a
non-analytical experiment, real world chemistry generally gives you yields of 65-85% of theory once all the purification steps are done.
Oh and Bonome, I hope that you aren’t trying to nitrate all that toluene, that picture is bothering me what would you need THAT MUCH TNT FOR ???.
(and even if you have a legitimate reason, do you think posting a picture like that along with the commentary you gave is wise??).
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I have seen mention in a patent of using a dichloromethane-nitric acid mixture to nitrate toluene and friends. It actually says it is reactive enough
to nitrate toluene at *-55C*. No other details were given for that specific reaction, but the patent did also say that it was gentle enough to nitrate
phenol or 9,10 dihydroanthracene with very little oxidation and thus very pure products.
Just an idea, but I think it might be effective, maybe even easier, to produce TNT by mixing toluene with the DCM-Nitric mixture, you might need some
concentrated sulfuric to suck up the produced water. The DCM shouldn't take part in the reaction and since water and sulfuric acid are not soluble in
it, it would basically be 100% nitric acting on the toluene. According to the patent you wouldn't need the high temperatures usually used. I would
guess this would result in substantially more pure TNT.
I was just thinking it could possibly be a good one step method that would require minimum quantities of acids.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
| Quote: | Originally posted by markgollum
Oh and Bonome, I hope that you aren’t trying to nitrate all that toluene, that picture is bothering me what would you need THAT MUCH TNT FOR ???.
(and even if you have a legitimate reason, do you think posting a picture like that along with the commentary you gave is wise??).
|
It really might be more appropriate to use a PM for commentary or questions regarding usage, functionality, or etiquette.
|
|
|
Fashist
Hazard to Self
 
Posts: 73
Registered: 19-7-2007
Member Is Offline
Mood: Powerful
|
|
| Quote: | Originally posted by CD-ROM-LAUFWERK
my TNT did melt around 81°C, wich is pure
the crackling is coming from entraped water in the TNT, u should meld it in a boling water bath and remove the water floating on top of it
than slowly cool it down, let the DNT crystallise out and pour the still liquid TNT out
repeating this two or three times will purify ur TNT to a mp. >80°C (if u make it properly)
|
May you Explain More ?
Interesting Method.
Thx
Chemistry=Chem+ is+ Try
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
In the final Step The Problem Is Water.The nitration will not Complete if Density of You Nitric acid Lower than 90%.For Solve This Problem You Have
Two way:
1-Use Oleum(The Amoung Of Oleum is very Important.Each Mole of Dnt need One Mole nitric acid And Need just one Mole of So3 No More!)
2-Use More Conc.Nitric acid(Use 3 Mole Conc.Nitric Acid for Every Mole DNT)
Pilot Plan For Making Tnt

[Edited on 17-3-2008 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
| Pages:
1
..
3
4
5
6
7
..
13 |