Hockeydemon
Hazard to Others
  
Posts: 218
Registered: 25-2-2013
Member Is Offline
Mood: No Mood
|
|
Any creative use for kavalactones?
My roommate recently returned from Hawaii and brought me home some local hippy drug. It's a perfectly legal substance - he bought it from a touristy
thing.
Anyways he brought me kava (waka grade). It's some god awful tasting stuff they drink as a ceremonial tea that has similar effects to being stoned but
it has the upside of most likely being carcinogenic.
I don't really have a use for it because I don't want to consume it. It is comprised of 6 different kavalactones so I would need to run it through a
column to separate the different lactones out. There is one main lactone called Kavian, and then an interesting lactone called Methysticin.
Methysticin:
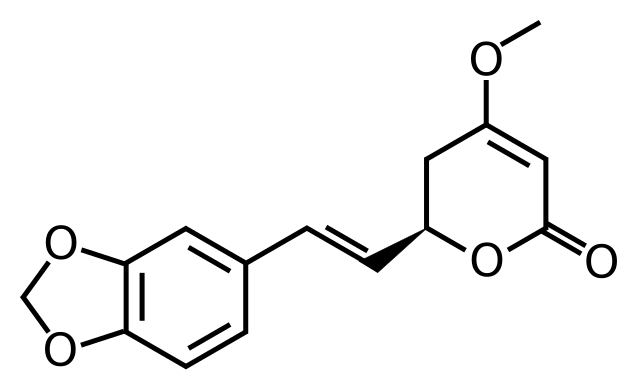
Kavain:

I don't know that there is much use for it. I honestly would be pretty happy making toluene from it. I do think it's pretty neat that if there is a
way to cleave that middle C=C pi bond in each compound you're not far off from isosafrol and amphetamine.
It would be nice if you humored me in telling me how impossible or possible getting to isosafrol and amphetamine from these molecules is, but I'm not
looking to actually attempt it. I would like to know why it would or wouldn't work in theory though (i.e the pi bond on the oxygen would be far more
reactive than the middle C=C pi bond).
Either way I would most likely use my universities facilities for whatever possible use there is since I don't have a column for chromatography. I
have about a 1lb of this stuff so it would be cool to find a use for it.
|
|
|
Mesa
Hazard to Others
  
Posts: 264
Registered: 2-7-2013
Member Is Offline
Mood: No Mood
|
|
The effects are completely dissimilar to being stoned. Neurological function is not impaired, there is no paranoia or related mood altering effects,
and there is no evidence whatsoever of kava being a carcinogen. There are reports of liver damage from long term use though.
As for what to do with it; I vaguely recall some people successfully smoking the extracted products. Although I'd implore you to try it in the same
way the natives would.
Ref: My name is Semesa, which is the Fijian equivalent of James. I've been drinking kava in the traditional Fijian manner weekly for the past 15
years.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
High tide or low tide? 
| Quote: | | I honestly would be pretty happy making toluene from it. |
Most crazy idea on this board ever...!
|
|
|
Hockeydemon
Hazard to Others
  
Posts: 218
Registered: 25-2-2013
Member Is Offline
Mood: No Mood
|
|
| Quote: | Several kavalactones (e.g. Flavokavain B, Methysticin and Yangonin) have been reported to be toxic and/or carcinogenic, although further research into
these mechanisms is needed and it is not yet known if kava consumption induces toxic/carcinogenic effects in vivo. Despite this, hepatoxicity has been
reported in a small portion of previously healthy kava users.[2][3]
Numerous kavalactones have apoptotic effects on various human tissues, which may be involved in some of the purported toxic effects of kava
use.[4][5][6] |
The lactone yangonin binds to the CB1 receptor and mimics the effects of THC. Kavain binds to the GABAA receptor which is the allosteric binding site
for benzos, ethanol, and barbiturates.
My buddy made me one cup and it was like drinking muddy water but with a poor after taste. Truthfully I didn't finish the whole thing. So I extracted
the lactones using IPA and dropped the extract onto a red-hot titanium nail. Boom instantly lethargic. I feel like if it was fun it would be illegal.
| Quote: | | Most crazy idea on this board ever...! |
Hey that's what I'm here for. Though this will probably get thrown in the beginning sub - humor me. Out of every functional group on the Kavain
molecule wouldn't that benzene ring be among the least reactive? Couldn't I reduce the lactone to a diol and use some H+ to remove the OH groups then
some how cleave off the unwanted alkane?
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
My doctor recommends Kava as an anxiolytic. Awful tasting isn't it?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
forsh
Harmless

Posts: 18
Registered: 12-11-2013
Member Is Offline
Mood: No Mood
|
|
Not very OTC, but throwing it out there ...
How about an oxidative cleavage to aldehyde or carboxylic acid? Osmate and Ruthenium salts do this nicely. Potassium permanganate possibly, but its
an ugly beast. Could do the Knoevengel condensation on the aldehyde.
I'm sure theres a few things you could do after an oxidative cleavage but I can't think right now.
:EDITED:
[Edited on 4-3-2014 by forsh]
|
|
|
Hockeydemon
Hazard to Others
  
Posts: 218
Registered: 25-2-2013
Member Is Offline
Mood: No Mood
|
|
I have plenty of potassium permanganate, but I don't have any osmate or ruthenium salts.
|
|
|