| Pages:
1
..
5
6
7
8
9 |
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
The hydrolysis step appears to be easier than anticipated. The reactor was fitted with a plastic funnel, cooled in an ice-bath and 3 x 80 mL H2O was
added in a thin stream. Each time, the reaction was allowed to proceed until the slurry seen in the funnel stem became too thick to allow gas out
easily. This slurry was collected in a 1 L beaker. The next portion of H2O was added, etc. A smell was noticed. It could be from a gas or a
microscopic mist. I think this should be enclosed when being done and a long period allowed for all possible mist to settle out.
Shown are a few seconds of hydrolysis 1 and pictures of #1, #2 and #3 which show decreasing reaction rates.
Attachment: 2015-09-24 10.28.23.mp4 (7.7MB)
This file has been downloaded 703 times   
[Edited on 24-9-2015 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
A couple things learned in the trial run w/o ThO2:
a) The Ca(OH)2 formed is a real nuisance. Now I understand what was implicit in the patents, that it is not dissolved away (solubility = 1.7 g/L @ 20
C), but rather poured of with the supernatant liquid. This explains why the thorium is obtained in two forms, heavy grains which settle much faster
than Ca(OH)2 and in the form of "fines" which are decanted off with much of the un-dissolved Ca(OH)2. This is a further handling/isolation issue to
address once the majority of the Th is recovered.
b) The presence of oxidation on the 1/4" NPT threads of the pipe which screwed into the reactor indicates some oxygen diffusion. This led to two
decisions. The first will be the inclusion of a minimum practical amount of a 2600 F nickel platelet/graphite anti-seize lubricant on the outer 1/2 of
the threads. The second has to do with the 1/4" NPT to 1/2" NPT bushing used for the intended reactor. The bushing is rust-resistant steel, not
stainless. I want it gone. So, I cut the 1/4" male NPT threaded end off of where I welded it to the pipe, and used a 1/4" NPT pipe die to cut threads
in it. I had a solid 1/2" NPT 316 SS pipe plug, with very high quality threads. I bored a concentric hole through the middle and will thread this with
a 1/4" NPT tap. After very tightly screwing this together, it will be welded all the way around with Weldmold 880 (a superb welding rod, about 8 to 10
times the cost of SS rods, but what an incredible performer!). This weld won't come in contact with the reaction mixture, so the high alloy content
won't worry me.
I have very nearly addressed every single mechanical aspect of doing the reduction cleanly.
One thing I wonder about, though. Since both the Ca and the Ca(Cl)2 will be molten, and the reactor will be turning at an angle and contains 3 large
Macadamia nut sized 309 SS lumps for stirring, why are Ca shavings and powdered Ca(Cl)2 needed? It would be preferable, from the standpoint of
moisture exclusion, to add cleaned Ca lumps and fused pieces of Ca(Cl)2 directly to the reactor. The mild "ball-milling" which will take place ought
to homogenize the reaction mixture. Any thoughts on why this may not be desirable?
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I can't think of any reason why you need to use powder/granules if the result is liquid.
I am vicariously loving this saga. Thanks for keeping us informed even though not many of us are able to contribute much.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Well, a few mundane but useful facts were learned:
a) Complete work-up of the blank, run in SS 304, gave only a few milligrams of insoluble gray material (perhaps too small to even collect and weigh).
This bodes well for minimal metallic impurities in the product.
b) Without a thread sealant, no amount of torque can seal the threads entirely gas-tight. My one concern is the hydrocarbon and graphite that will
burn off the sealant and cause minimal C contamination of the Th. If that's the case, it still greatly beats O2 contamination.
c) I used 1.5 times the specified amount of dilute HNO3 to dissolve all of the Ca (OH)2. If some Th is lost, it will be (recovered) worth it to avoid
solid-bearing mists from high speed agitation.

"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Not surprising. Even though NPT are considered fluid-tight it requires exceptional tolerances or that one part can yield. If a sealant isn't desirable
a gasket or crush washer between the two nuts should work.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
I've thought about those options, but I didn't have any bright ideas on what to use.
That area will be in air for a minimum of 1 hour, and ideally several hours, at something approaching 1000 C. That rules out so many options that it's
ridiculous.
And given that it needs to be soft enough to deform sufficiently to become gas-tight....tough nut to crack.
I think very, very thin platinum foil could work as a Teflon tape substitute, something along the lines of cheap brands of aluminum foil.
The other sealant I considered was MoS2. It has a very high mp but the literature says:
Ambient Temperature Range -185 to 350 ºC
Vacuum Temperature Range -185 to 1100°C at 10-14 Torr
So, it may not be the best option.
The graphite/nickel platelet formulation claims a 2600 F rating. I plan a few experiments where I put the mix on male threads, bake it at different
temperatures, and compare the sealing properties of the baked mixture with the normally applied paste.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
That is some serious conditions, bro. But does it have to be completely oxidation proof? Only the outer surface will oxidize, so as long as it's
diameter is large some loss shouldn't affect the seal. And any oxygen leaking through the mating surfaces would oxidize the material, wouldn't this
produce an increase in volume that could cause it to be self sealing. Not sure if that would work, but it's worth some consideration.
One other problem with a gasket like this is the thermal expansion, if it is different from the vessel it could cause problems either during heating
or subsequent cooling. So a thin gasket would probably be the safest option.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Texium
|
Thread Moved
9-5-2016 at 18:28 |
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Tomorrow.

"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
YAYYY!!! Such a fun project to follow  thanks for sharing thanks for sharing
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Thorium production moved to Radiochem.
This move was surprising, in that radiochemistry is merely one of the characteristics of the desired product. This is a synthesis topic. Always has
been.
And it will happen tomorrow.
http://www.sciencemadness.org/talk/viewthread.php?tid=29927&...

"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Agreed. Surprising. Following this I think that the major challenges have been engineering ones to cope with the extreme nature of the chemistry
involved. It makes as much sense to put it in technochemistry as it does in radiochemistry.
(Shouldn't this thread be in Forum Matters?  ) )
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Sorry, I moved it without thinking much of it. I'll put it back in Chemistry in general and merge this to it. No problem.
|
|
|
Texium
|
Threads Merged
27-7-2016 at 20:02 |
Texium
|
Thread Moved
27-7-2016 at 20:02 |
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Thank you, zts 16.
And now, on with the show.
I'll prepare a detailed write-up to accompany this prep.
For now, here are some visuals:
https://www.dropbox.com/s/w7p1lr0m47dduky/Start%20of%20Th%20...
This is a short clip of the start of the reduction (initial heating).
I'm now at 950 C (2:20 PM).

"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Dan:
Tell me again what is the purpose of the inclination?
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
It's to achieve good mixing. If it were vertical, stirring wouldn't be as good. The original workers milled their charges to get a homogeneous
mixture. That wasn't easy, practical or safe for me.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Quote: Originally posted by Fulmen  | That is some serious conditions, bro. But does it have to be completely oxidation proof? Only the outer surface will oxidize, so as long as it's
diameter is large some loss shouldn't affect the seal. And any oxygen leaking through the mating surfaces would oxidize the material, wouldn't this
produce an increase in volume that could cause it to be self sealing. Not sure if that would work, but it's worth some consideration.
One other problem with a gasket like this is the thermal expansion, if it is different from the vessel it could cause problems either during heating
or subsequent cooling. So a thin gasket would probably be the safest option. |
A practical metal gasket for threaded connectors? I didn't know they existed. Funny you should mention oxidation though..."heat-resistant" SS 308
seemed to fare far worse at 950 C than the SS 304 that I usually use. That forms a tightly adherent scale, this eroded away layer by layer. I was
certain that I had a reactor breach upon first sight. I'm still inspecting and cleaning the reactor and struggling greatly to unscrew the connecting
pipe.
  
[Edited on 29-7-2016 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@Dan:
Thought as much. Thanks and good luck!
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
I cleaned thing things up in preparation for hydrolysis in place. Mostly okay, but one area is slightly suspicious. Still assessing. Yes, I know this
is glacial. Sorry, but it is progressing....
  
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
The 1/2" threaded connection yielded to a pipe vise and a 24" pipe wrench.
The reactor was prepared for the "in-place" hydrolysis by thoroughly isolating the reactor's exterior to prevent sloughing off of impurities during
submersion. Theoretically the interior should be quite clean.

"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
The interior, what I could see of it, was clean. Hydrolysis was performed as per 9-24-15 trial run, but under a sophisticated cover - a clear acrylic
wastepaper basket with a 1/8" hole in the top, er, bottom...you know what I mean, for drop-wise distilled water addition. I'm eating spaghetti, it's
ever so slightly effervescing. Tomorrow morning I'll pour it out....
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Well, it's done...or getting there. But there's the thorium.....
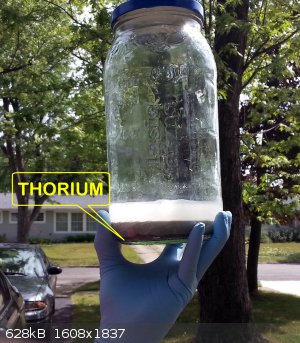
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Extraordinary thread, just finished reading it all. Bit like a decent book in places, a few twists and turns 'would he bottle it or would he not'.
No idea how dangerous that stuff is but i got the impression its not something to be messed with, so seeing a jar held aloft by hand at the
end............I thought 'now thats what you call a utterly MAD scientist!!
I am fully expecting you to be supplying your neighbours next year with self generated nuclear power.
Well done its beyond impressive which ever way you look at it. Why does everything great always look like sludge? Only stuff like sodium chloride
seems to look pristine white and fluffy.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
I had the same thought, it's a bit anticlimactic to look at.
Thorium is like most primarily alpha emitters. Essentially harmless in the sense that alpha particles don't penetrate skin. But ingested or inhaled
alpha emitters are super dangerous, see polonium, plutonium.
Now, on to the final isolation...
[Edited on 2-8-2016 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dan Vizine  | I had the same thought, it's a bit anticlimactic to look at.
Thorium is like most primarily alpha emitters. Essentially harmless in the sense that alpha particles don't penetrate skin. But ingested or inhaled
alpha emitters with long half-lives are super dangerous, see plutonium.
Now, on to the final isolation...
[Edited on 1-8-2016 by Dan Vizine] |
Must be deeply satisfying though after all the effort to finally get your crude product.
Well done its a really inspiring read.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Thank you most sincerely.
The story isn't over by a fair stretch. A good write up needs to be done, all info. in one succinct spot. Anomalies (there was one, as hinted at by
the reactor's bottom edge) need analysis. Spoiler..it seems to have been rather benign. And the final product isn't a final product until fully
processed, washed, dried, passivated & bottled.
Yes, satisfying and a relief. So many man-hours, so much labor and more than a few $$ were involved. I even built the furnace for this project. The
tank of UHP argon was $140, the tig welding was $140, the chemicals were over $800, misc. materials were another $50. Way, way over 100 man-hours all
things considered. Perhaps 200.
[Edited on 1-8-2016 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
| Pages:
1
..
5
6
7
8
9 |