| Pages:
1
2 |
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Methylenediisonitramine
Me and Rosco talked of this before, but I've only just got around to trying it, since its synthesis is closely related to those in the <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=2960">pseudonitrosite thread</a>, but to avoid taking that thread too far off
topic, its here.
<center><img src="http://www.sciencemadness.org/scipics/axt/medna.jpg"></center>
Methylenediisonitramine (MEDNA) isn't exactly obscure, as its referenced in both Urbanski and PATR 2700, also in US1625966. Its interesting that it
hasn't made mention, being isomeric to MEDINA it should be a very powerful explosive, MEDINA being one of the most powerful explosives known, with:
VOD = 8700m/s
Ballistic mortor = 198% of TNT
Trauzl test = 681-727cc
Sand test = 71.9g (RDX = 58-61g)
Though sadly the references don't report on any performance properties for the subject of this thread. Both MEDNA and MEDINA possess perfect oxygen
Balance, as do their salts:
CH<sub>4</sub>N<sub>4</sub>O<sub>4</sub> → CO<sub>2</sub> + 2 H<sub>2</sub>O + 2
N<sub>2</sub>
The problem with MEDNA is that it can only be isolated as its salts, greatly limiting its usefulness. US1625966 and Urbanski recomend the lead salt as
a component in detonators, which in combination with lead styphnate seem to show better initiating properties the lead azide. Though when ignited by
itself doesnt DDT readily enough to be used alone in detonators. PATR seems to suggest that its thallium salt can be used alone.
<u>Minimum quantity to initiate tetryl</u>
Lead azide / lead styphnate 80:20 = <b>0.15g</b> (Urbanski)
DDNP / potassium chlorate 75:25 = <b>0.25g</b> (Urbanski)
Lead MEDNA / Lead styphnate 70:30 = <b>0.09g</b> (US1625966)
The references give its synthesis by the interaction of nitrogen oxide with acetone in the presence of sodium ethoxide. I wanted to try the synthesis
without anhydrous conditions, rather using a saturated solution of EtOH and NaOH.
CH<sub>3</sub>COCH<sub>3</sub> + 4 NO + 2 C<sub>2</sub>H<sub>5</sub>ONa →
CH<sub>3</sub>COCH(N<sub>2</sub>O<sub>2</sub>Na)<sub>2</sub> + 2
C<sub>2</sub>H<sub>5</sub>OH
CH<sub>3</sub>COCH(N<sub>2</sub>O<sub>2</sub>Na)<sub>2</sub> + H<sub>2</sub>O →
CH<sub>3</sub>COOH + CH2(N<sub>2</sub>O<sub>2</sub>Na)<sub>2</sub>
Experimental: A saturated solution of sodium hydroxide was made by boiling NaOH with EtOH, cooling and filtering. 90ml of the filtrate was added to
10ml acetone in a 100ml measuring cylinder.
A NO generator was made by adding 2 lengths of 15mm copper pipe into a 250ml flask and injecting 100ml nitric acid diluted with 150ml water. The NO
was allowed to bubble through the EtOH/acetone/NaOH solution for 3 hours.
After three hours the solution had turned a peach colour, and a precipitate of yellow crystals formed, which should be the sodium salt of MEDNA. The
sodium salt had a great affinity for glass, sticking to it thus couldn't be filtered. Rather the liquid portion was poured off, and water was added to
dissolve the salt, which dissolved readily. Into this solution was added a solution of AgNO3/water, which precipitated an orangey flockulent
precipitate which quickly turned black. This was filtered and dried in the sun. It seems that anhydrous conditions are only used to facilitate
precipitation of the sodium salt, as its almost insoluble in ethanol.
The dried silver salt burns fast in the open, at rate simular to blackpowder, no detonation has been attempted.
<center><img src="http://www.sciencemadness.org/scipics/axt/silvermedna.jpg">
<a href="http://www.geocities.com/roguemovies7/index.html">MOVIE</a></center>
By substituting acetone with nitromethane a different explosive is formed, nitromethylisonitroamine CH3N3O4, OB = +6.61, Urbanski mentions that salts
of this explosive are weaker when used as initiators then MEDNA, owing to the nitro group. I have not attempted this explosive, nor do I know if its
isolatable in its pure form. Esters of MEDNA and the nitromethane derivative may be interesting.<br><br>
[Edited on 9-12-2005 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I tried something else yesterday, I tried to form a salt by reacting Cu pipe directly in a mixture of dilute HNO3/acetone. The hope was that MEDNA
would form and preciptitate directly from the copper nitrate as its Cu salt.
The reacting went nicely, with the Cu pipe giving massive surface area for the reaction to take place. It dumped a light green insoluble precipitate,
when dry the copper salt was only feebly explosive, smouldering away leaving black residue.
Hmmm, amazed that something happened at all, I tried silver and mercury metal in the same setup. They bubbled away releasing NO, but as the reaction
proceded they suddenly erupted in foam and NO2. Some product was left, in both cases it was yellow and insoluble. The silver salt darkened in
sunlight.
When ignited they were explosive, but not as energetic as the MEDNA salt, flaring off in a crackling flame. I've no idea what these salts are, but it
would be interesting to try variations of this with other reactants, such as nitromethane.
<center><img src="http://www.sciencemadness.org/scipics/axt/a_silversalt.jpg"></center><br><br>
[Edited on 9-12-2005 by Axt]
|
|
|
mark
Harmless

Posts: 25
Registered: 23-10-2004
Member Is Offline
Mood: No Mood
|
|
I had recently attempted it using the following.
45 ml of saturated EtOH and NaOH solution was added to 5 ml of acetone and stirred in. This was poured in to a 100ml-graduating cylinder. NO/NO2 and
other NOx gasses were bubbled through this solution for some time. The generator was copper pipe in a nitric acid (8 molar) solution. The solution
was now added to AgNO3 (0.1 molar) until a large amount of black precipitate was formed. It was then filtered. This precipitate seemed to be
absorbed into the paper. It doesn’t come off? Did you have this problem?
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Solution to your problem ... make more, the copious sludgy precipitate will shrivel up when dried.
Reading your description, theres a good chance that you have silver oxide rather then the MEDNA salt. Dry the paper and ignite it, then you will
know. I only used the precipitate and not the solution.
[Edited on 18-12-2004 by Axt]
|
|
|
mark
Harmless

Posts: 25
Registered: 23-10-2004
Member Is Offline
Mood: No Mood
|
|
Slightly disheartened by the previous attempt I decided to try AXT second method.
The reaction in my attempts has 2 possible out comes. One being a “runaway” were it froths up and NO2 gas is spewed out. And were the acid is
diluted, a small bubbling of the copper happens and the mixture turns the colour of CuNO3 solution but no precipitate happens? I have attempted this
reaction 4 times and 2 reactions frothed up and the other 2 proceeded lazily with no product. I am using 8 mole nitric and pure acetone. So what
dilution is your nitric acid gone to?
Before bubbling out NOx
http://www.infernolabs.co.uk/filehost/PICT1.JPG
After Bubbling out NOx
http://www.infernolabs.co.uk/filehost/PICT0005.JPG
[Edited on 19-12-2004 by mark]
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I only tried once with the three metals, the copper salt yield was the smallest of the lot, even without it erupting (maybe it should erupt to
complete the reaction). I didnt measure the reactants, as I wasn't expecting anything to happen at all.
Using the method in my second post, the copper salt is so feebly explosive its worthless. The Ag & Hg salts arent useful either, just a curiosity.
I tried substituting acetone with NM, but nothing precipitated. Though NM/dilute HNO3 seemed to attack Ag mighty fast.
[Edited on 19-12-2004 by Axt]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Carefully read US1625966 page 1 lines 53 to 81
The salts of " iso-MEDINA " are not
" unequivocal " primaries like silver fulminate . That is to say their capacity for detonation from self-accelleration is
poor , and the materials simply burn when initiated by fire . The behavior is
similar to tetracene and a few other materials which are more valuable as mixtures , where the complementary properties of each component combine to
yield a " synergistic " binary composition .
Therefore , in order to realize the potential power of the lead salt of
" iso-MEDINA " , it * must * be mixed with another " igniter " like lead styphnate or basic lead picrate . Probably
lead nitrato-bis-basic picrate or the non-azo-clathrate , basic lead picrate / lead nitrate / lead chlorate would work equally well as " flash
igniters " to compensate for
the poor self-ignition behavior of the lead
salt of iso-MEDINA when used alone . Possibly it would require some of the loose flash igniter or some of the mixed composition atop the pressed
material to
provide the start of the detonation sequence as a low order DDT which would
then accellerate to high order detonation in the pressed binary composition . But it is equally possible that the pressed binary composition would
DDT without any need for a loose top charge . Only tests would reveal the behavior of the binary in this regard .
Such complementary mixtures and composition specific firing schemes are not uncommon .
Anyway , it is clear from the patent that no clue can be gotten from the behavior of the material to simple ignition , concerning the energy output
which is revealed under conditions favorable for its high order detonation . Further tests would be needed to see what the binary compositions do
when initiated under confinement , to confirm or dispute the usefulness of the composition reported by the patent .
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
How about MEDNIA - methyl dinitramide? It has close to zero oxygen balance and should be more stable than MEDINA, because it can't hydrolyse to
nitramide which then decomposes to form more water which accelerates the process (the same thing happens to urea), while still being very powerful? It
could probably be synthesized by reaction methylamine with a nitryl salt.
|
|
|
Joeychemist
Hazard to Others
  
Posts: 275
Registered: 16-9-2004
Location: Canada
Member Is Offline
Mood: Sedated
|
|
MEDNA will form explosive salts with; Barium, Cadmium, Calcium, Cesium, Copper, Iron, Lead, Mercury, Potassium, Rubidium, Silver, Sodium, Thallium,
and Tin. I have read MEDNA will form a salt with Monohydroxylamine but I’m not sure.
I will hopefully be attempting the Sodium, Calcium, Lead and Barium salts this weekend. I finally found a use for 2½g of barium. 
To synth the Barium salt react Barium Chloride in a solution of the (Na)MEDNA salt. Filter and dry the precipitated (Ba)MEDNA. The Barium salt is
noted to be the weakest of the MEDNA salts.
The Calcium salt is formed in the same way as the Barium salt. Calcium Chloride is added to a solution of the Sodium salt and the precipitate is
filtered and dried.
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Rosco Bodine
Therefore , in order to realize the potential power of the lead salt of
" iso-MEDINA " , it * must * be mixed with another " igniter " like lead styphnate or basic lead picrate . |
In another thread, whichever it was I expressed my interest in trying to couple a diazonium salt onto MEDNA in order to give it the "briskness" to
DDT. Attempts to diazotise nitroaniline in acetone/alcohol with and without acids with NO have thus far failed to produce anything of worth. The
convenience of a one pot reaction between acetone/nitroaniline/Nitric oxide to create nitrobenzenediazonium-MEDNA, where it would have to undergo
quite complex reactions in a mixed solvent hasn't been realised.
I was also unable to form a precipitate from nitrobenzenediazionium sulphate and sodium salt of nitromethane, on mixing it seemed to produce a very
fine orange precip, but on standing for a couple minutes the solution turned a transparent red.
Note that if one searches google for ""benzenediazonium salts with nitromethane" you get a unopenable (for me) journal article that mentions a diazo
coupled product of aniline/NM.
Anyway, since this topic is back on top, heres two german articles detailing methylenediisonitrosamine that I've aquired.
MEDNA 1 - http://www.sciencemadness.org/scipics/axt/methylenediisonitr...
MEDNA 2 - http://www.sciencemadness.org/scipics/axt/methylenediisonitrosamine(ber).pdf
Joeychemist, the original ref. for the MEDNA salts is PATR, which gives more detail. Though the ref. it gives is an obscure urbanski publication.
Their effectiveness in the Pb block test is as such:
Ba -> Cd -> Cu -> Fe -> K -> Na -> Sn -> Tl
No comparisons to its Pb or Ag salts are given, nor any to any conventional explosives which is a pitty (no exact figures are mentioned either).
I've only recieved quite pittiful yields via NaOH/ethanol/acetone, so dont expect much. The Pb salt deflags slower then Ag, but dets readily under the
hammer.
[Edited on 9-12-2005 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Attached is an extract from an article titled "The Ultraviolet Absorption Spectra of Aliphatic Nitramines, Nitrosamines and Nitrates" Its
related to determining the difference in spectra between MEDINA & MEDNA. This isnt of great practical value to us, but one sentence caught my eye
which states:
<i>"The Traube compound is unstable in the free state and is usually prepared as the mono-ammonium salt or the disodium
salt."</i>
One of the German articles above gives the syth of the ammonium salt, however derived from its Ag salt, this route has little value. The above quote
suggests that the ammonium salt can be made without going through the sodium, silver salts, NH<sub>4</sub>-MEDNA should be a useful HE.
Only known comparisons are with the MEDINA salt:
NH<sub>4</sub>-MEDINA is listed in PATR as being 142% power of TNT, where MEDINA is 154-158% and RDX is 150% on the ballistic mortar test.
(Vol. 2, B 266)
Experimental:
Into 80ml acetone in a 100ml measuring cylinder was bubbled 100ml 70% HNO3 worth of NO (though tubing was leaking) by the reaction with copper pipe.
The solution remained clear and seemingly unchanged.
The copper nitrate solution was poured out, and flask charged with ammonium nitrate and sodium hydroxide as to generate ammonia gas. This gas was fed
into the acetone solution and an immediate white precipitate formed, hopefully the ammonium salt of the intermediate of MEDNA, but possibly just
NH4NO3.
The Acetone was decanted, water was added and the precip dissolved readily. This was then precipitated with Pb acetate to make sure that it was not
NH4NO3 (from HNO3 fumes coming over from the gas generator). Since it precipitated, it wasnt a nitrate salt. The lead precipitate is now drying
(though it was just rained on  ) )
Hopefully it will show the properties of Pb-MEDNA, thus giving a higher yielding syth for both the secondary HE from ammonia and the primary from lead
salts.
[Edited on 17-3-2005 by Axt]
Attachment: medna-spectra.pdf (664kB)
This file has been downloaded 1380 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Something I have been thinking is that since it appears anhydrous conditions
are not essential , it may be possible to do the reaction even more simply . What I have in mind is dissolving lead nitrate or lead acetate in moist
acetone containing some acetic acid , even vinegar , or dilute nitric acid , so that the acidic acetone solution has sufficient H20 for solution of
the lead salt . Then add sodium nitrite as a saturated aqueous solution for the nitrosation which would occur as a result of reaction
with the acid content of the mixture , generating N2O3 in situ . The lead salt of MEDNA might precipitate directly from that one pot reaction .
In regards to generation of nitrous gases ,
Microtek has reported success generating N2O3 from decomposition of medium concentrated nitric acid on mixing with starch and warming . I can't
find the reference , but paraformaldehyde is supposed to do the same thing .
[Edited on 17-3-2005 by Rosco Bodine]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Here is the reference that Rosco was refering to:
"43 ml HNO3, 50% is added to a flask along with about 10 g potato starch. The flask is equipped with a one hole stopper wit a hose inserted (
both hose and stopper should be somewhat acid resistant; PE will do ).
The flask is heated until the reaction becomes self-sustaining and the mix of NO and NO2 which is in equilibrium with N2O3 is bubbled through..."
From p6 of "My favorite primary explosive" thread, posted by Microtek
http://www.roguesci.org/theforum/showthread.php?t=3158&p...
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Nearly pure Nitric Oxide , ( NO ) , can also be prepared by heating a mixture of K nitrate , Ferrous Sulfate , and dilute sulfuric acid . PATR , vol.
8 , page N-129 .
2 KNO3 + 5 H2SO4 + 6 FeSO4 ---->
3 Fe2(SO4)3 + 2 KHSO4 + 2 NO + 4 H2O
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
a nitric oxide preparation using sodium nitrite instead
2 NaNO2 + 2 FeSO4 + 3 H2SO4 ------>
2 NO + Fe2(SO4)3 + 2 NaHSO4 +
2 H2O
The reaction details are in the file attached and found at the following page
http://services.juniata.edu/ScienceInMotion/chem/labs/gases/...
Attachment: noxs.doc (46kB)
This file has been downloaded 1281 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Just a thought .
Acetone Peroxide Trimer dissolved in toluene or another solvent , may possibly react with nitric oxide in a similar way as does acetone and lead to
the same or
a different but related compound .
I haven't tried this reaction so I don't know and this is purely speculation .
This could be an interesting experiment .
The idea occurred to me after the reaction of propylene oxide was described in the other thread . There is just a chance that a cyclic product might
result if trimeric AP does react with nitric oxide . If so , the resulting product could have increased power and stability , and would possibly also
be a newly discovered compound if it does indeed exist .
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
AP was dissolved in xylene in which it seems very soluble. Into 60ml of this strong solution of AP was passed N2O3. Solution turned dark green then
temperature shot up and seemed to undergo violent decomposition, erupting with orange fumes leaving about 30ml of an orange solution.
N2O3 was then passed through straight xylene, it quickly turned dark green... then I run out of N2O3.
.... So, it does seem to act on AP, but xylene is too reactive to use as a solvent to know what you end up with.
Ether, DCM & hexane are possible solvents which should both dissolve AP and be inert towards nitrogen oxides. Hopefully one will also precipitate
an addition product.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
These reactions remind me somewhat of chlorination reactions . It may be that specific NO or NO2 may be required for some reactions instead of the
N2O3 that
may work for others . For peroxides it may be that NO alone is necessary. What I was thinking is that the NO might react with the existing peroxy
groups and convert them to ONO2 or NO3 groups without disrupting the existing ring structure . It may be that cyanuric acid is another potential
cyclic compound of interest for nitrosation . Also hexamine or hexamine dinitrate or even HMTD could form R-salt or a similar derivative . Urea seems
another less likely possibility , but may simply decompose to nitrogen and water . MEK is another possible precursor of interest . Naphthalene is
another . Acetone formaldehyde or methylol condensates are also possible precursors for nitrosation . Just about anything that could be nitrated or
chlorinated is a candidate for nitrosation .
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Another thread
https://sciencemadness.org/talk/viewthread.php?tid=2656
concerning sodium ethoxide from heated ethanol and excess NaOH has relevance to the initial experiment
of this thread . This could have bearing upon the yield .
Also it may be of benefit to have a higher percentage
of acetone in the mixture with the alcohol and sodium ethoxide , and use a tall enough bubble column and fine enough gas bubble dispersion that few
bubbles reach the
surface at the top of the column to escape unreacted .
When the experiment was done , how efficient in this regard
did the bubble column seem to be operating ? Also I have
to guess that there is an optimum temperature and height combination for the column which is ideal and would favor good yields .
The true value of these initiators is evidently their ability to
form coprecipitated mixed crystals ( possibly double salts? ) with basic lead picrate , normal lead styphnate and other similar low order primaries
which are not by themselves alone powerful enough for use in any reasonable quantity to initiate insensitive base charges , yet when combined with the
lead methylene diisonitroamine , form a synergistic
* coprecipitate * which weight for weight is a better initiator than lead azide , and safer and more stable than lead azide . It seems possible also
that a series of
" diisonitroamine-clathrates " could be formed , and this
could be an interesting line of experimentation .
For a high performance initiator , the technical difficulty would be less for the total synthesis of these type compositions than for compositions
containing azide ,
and the performance better , if they perform as claimed
by the patent .
Evidently MEK used instead of acetone leads to the ethylidene diisonitroamine , as the patent intimates
but does not openly disclose , that perhaps an equimolar
mixture of acetone and MEK can be used to obtain a
mixed methylene-ethylidene diisonitroamine product .
Von Herz does say that it is advantageous to employ these two substances together ....but gives no further details ,
which seems curious .
Also , after giving more thought to the nature of this reaction it seems to me that it is important for the initial reaction to have anhydrous
conditions for best yields . It would be a good idea even to run the nitric oxide through a drierite
or calcium chloride cannister before delivery to the dispersion
tube at the bottom of the bubble column , and use well dried
acetone , and carefully prepared sodium ethoxide in alcohol ,
keeping everything as dry as possible .
I am curious about the experiment with the ammonia salt above , and not sure what was produced ....except that it was probably something different
from the compound hoped to result . I can't quite work out that reaction so that the dots connect the way they do with the sodium ethoxide reaction .
[Edited on 2-9-2006 by Rosco Bodine]
Attachment: US1625966 methylene diisonitroamime initiator compositions.pdf (169kB)
This file has been downloaded 1311 times
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
When I done the above it wasn't done with efficiency in mind, it was just bubbled through a glass tube into a 100ml measuring cylinder. There is a
problem with trying efficient bubbling as the precipitate that I formed was quite sticky and would gum up small holes. Though this stickiness isn't
mentioned in the literature so may be a result of the conditions I used.
Interesting enough, the "isonitramines" have been given a new lease on life as the "diazeniumdiolates" or "diazene-N-oxide-N'-hydroxylates", and have
recently been given a lot of attention in the literature as NO donors for medicinal use.
It actually doesnt seem to be adversly effected by a bit of water released by NaOH, for example:
"To a solution of sodium hydroxide (6.0 g, 0.15 mol) in methanol (150 mL) was added acetone (2.18 g, 0.0375 mol). The solution was allowed to react
with nitric oxide as described above for 4 d at 20 °C. The white precipitate formed was filtered, washed with methanol (50 mL), and dried." JACS.
2001, 123, 10860-10869
Most interesting may be the methane tris(diazeniumdiolate) salts, which are oxygen rich. Even the trihydrated sodium salt is said to "detonate
violently when heated". Thus a Pb salt mixed with aluminium could prove to be an effective initiator. They are formed from the same reaction with
acetone but conducted under pressure. Also formed, in a more pure form, from NO/acetonitrile.
I can't confirm nor deny the ammonium salt formed when I did that above, (it was washed away!) literature seems to suggest that basic conditions are
needed.
Attachment contains:
N. Arulsamy and S. Bohle "Multiplicity Control in the Polygeminal Diazeniumdiolation of Active Hydrogen Bearing Carbons: Chemistry of a New Type of
Trianionic Molecular Propeller" J. Am. Chem. Soc. 2001, 123, 10860-10869
Ernst V. Arnold et al. "A Nitric Oxide-Releasing Polydiazeniumdiolate Derived from Acetonitrile" Org. Lett., Vol. 4, No. 8, 2002
Both are very interesting regarding the bis/tris(diazeniumdiolates).
[Edited on 2-9-2006 by Axt]
Attachment: diazoniumdiazolates (isonitramines).pdf (198kB)
This file has been downloaded 1712 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Thanks for the very interesting article .
Did you already have this article before starting this thread ?
It sheds more light on this interesting reaction and
somewhat resolves any confusion about the matter of the sodium ethoxide requirement by the declaration
that sodium hydroxide works just as well . But I wonder if it works just as well with other conditions being equal . It is terrible that the details
of experiments
which indicated the equivalency of NaOH with the ethoxide were not shared , but simply mentioned in passing .
That equivalency observation possibly destroys the original hypothesis for the mechanism of the reaction ,
and an added indication of this is provided that the reaction also proceeds in methanol . However , I have some question about whether that
equivalency is an unqualified observation . It could be that the reaction proceeds differently under different conditions . It may be that in an
unpressurized apparatus that the sodium ethoxide in ethanol is the best way to go as it would seem likely to be more reactive .
Also interesting that this is a fairly recent publication ,
from six years ago .
A side note here in the " thinking out loud " category 
I have been wondering if there might be a reaction between
trimeric acetone peroxide and R-salt in methanol , or if
R-salt dissolved in plain acetone might react with the solvent
on long keeping , perhaps at elevated temperature or
with some slight acidification or added catalyst . I keep thinking that some sort of nitric oxide donor in solution
would be more convenient than working with gas generators and bubblers , and could lead to the same or similar compounds . Organic nitrite esters
would also seem to
be a possibility here as such nitric oxide donors . Perhaps
even nitrosyl sulfuric acid , or some sort of aromatic nitrososulphonic acid? could have usefulness in some reaction scheme of this general sort .
There is a lot of food for thought and unexplored territory where these sorts of
reactions are concerned .
[Edited on 2-9-2006 by Rosco Bodine]
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
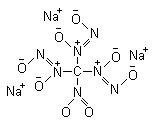
Nah, I only come happened across them a few weeks ago. Knowing what to search for helps, considering the powers that be decided on a new
name/structure.
Also considering how recently the reaction, if pushed, has shown to produce the tris(diazeniumdiolates). Wonder if its possible to push nitromethane
all the way to produce sodium nitromethanetris(diazeniumdiolate) CN7O8Na3. That'd be interesting.
[Edited on 2-9-2006 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Another article of relevance.
Attachment: Chemistry of the Nitric Oxide-Releasing Diazeniumdiolate- Chem. Rev. 2002, 102, 1135-1154.pdf (274kB)
This file has been downloaded 3323 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Page 4 of the article just above is saying exactly what I have been thinking as regards to nitrosyl " donor " scenarios ,
already thought about nitrosyl chloride too ......
and indeed one of the things I had thought of was adding
an organic nitrite to an acidified alcohol / acetone solution
at low temperature . Good stuff being added here with these new references . Hopefully this is leading to some refined methods . Reference #39
would be interesting there .
BTW , nothing like 16 pages of " light reading " having 302 references  ...... ......
my eyes began to cross and my brain
went into " scan mode " on about page 5 .....
How about the rest of you  ? ?
Nerds rule , that's for damn sure .
A rose by any other name ....
but indeed the nomenclature changes
get hard to follow when researching for the
old name , unless the change is conspicuously noted ,
and cross-referenced also to the old name in the
newer references . Also noticed the archaic " gr. "
abbreviation for *grams* in the older patent .
The #13 reference in the University of Wyoming paper
could be interesting also . I need to get a password for
the references thread . If anyone has access to that
Canadian Journal of Chemistry article please attach it here in this thread .
George , Kierstead , and Wright , Can. J. Chem 1959 , 37 ,
679-699
[Edited on 2-9-2006 by Rosco Bodine]
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Rosco Bodine
adding an organic nitrite to an acidified alcohol / acetone solution at low temperature. |
Dont think you'd have anything forming in acid solution. Unless I've read something wrong your target compound would be the one donorin'
Yeh, the chem. rev. article gives some other nice possibilities, particularly the N-diazeniumdiolates. Though I cant think of any easily accessable
secondary amines, I'd probably start at glyoxal-ethylendiamine condensation, then try react that in place of acetone hoping for .... eh, the damn ugly
sounding "sodium decahydropyrazino[2,3-b]pyrazine-1,4,5,8-tetrakis(diazeniumdiolate)". Really gotta think up a better name, PPTDD will do  or how'bouts tetraisonitraminopyrazinopyrazine. or how'bouts tetraisonitraminopyrazinopyrazine.
What other secondary amines are available and offer acceptable OB? most urea derivatives wont be soluble in alcohol.
[Edited on 3-9-2006 by Axt]
![decahydropyrazino[2,3-b]pyrazine-1,4,5,8-tetrakis(diazeniumdialoate).jpg - 8kB decahydropyrazino[2,3-b]pyrazine-1,4,5,8-tetrakis(diazeniumdialoate).jpg - 8kB](http://www.sciencemadness.org/talk/files.php?pid=74962&aid=1720)
|
|
|
| Pages:
1
2 |