CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Steam distillation
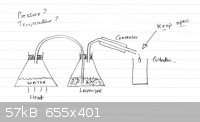
Wanting to set up Steam Distillation for my wife because Lavender does not do well in a water distillation setup. I have enclosed a diagram that
avoids all the expense of what you find for sale. I am new to this and do not even know the names of different glassware yet, so maybe someone might
be kind enough to perhaps help improve this setup (safety) or suggest different glassware if absolutely necessary, or a better design. I have
considered stainless steel kettles and urns (Make it myself) but very difficult to find any thrown out and embarrassing looking in everybody's skip to
see what is thrown away. (I found my heat chamber this way).
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
Muffn Man
Harmless

Posts: 45
Registered: 12-11-2013
Member Is Offline
Mood: No Mood
|
|
A few ideas
First off, to avoid embarassment of looking into skips:
http://www.fastcodesign.com/3030206/subversive-mask-fools-su...
Sort of just kidding. One man's junk is another's treasure!
Have you looked at second hand type stores? They are usually associated with charitable organisations. You can pick up big stock pots and the like.
You can build a cheap Liebig condensor with copper pipe and plumbing fittings. Just Google "diy copper liebig condenser" and you will find many
tutorials and pictures.
|
|
|
weeksie98
Harmless

Posts: 36
Registered: 24-10-2013
Location: England, UK
Member Is Offline
Mood: Pretty protic
|
|
Sorry to be completely off-topic, but you're an International Hazard, with 717 posts, and you don't know the names of the glassware?
'If organic chemistry were easy, it would be known as "biology".'
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Your setup looks pretty much the way it should. Some people would put a trap to only have pure steam coming into the material to be distilled but for
this purpose it isn't necessary. As far as safety goes it would be fine!
Weeksie: what exactly was the purpose of that comment? Sarcasm?
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Prolly missing something here, given that it's been a while since I've last had to steam-distill anything, but why aren't you using "internal" steam
distillation to get at the lavender oil? That is, immerse those flower spikes in a good amount of water in some flask, hook it up to a condenser, and
heat away. If you don't have the appropriate flasks, it should not be too difficult to jerryrig an equivalent setup with more available containers,
just making sure that everything that's sealed up is sealed up.
In any event, IIRC Zubrick has a nice basic discussion on this; you might want to try looking for his book.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
There was a question on the subject posted recently by another member who failed to use the search engine:
http://www.sciencemadness.org/talk/viewthread.php?tid=30445
in which a link to an old thread is given  Some more pertinent documents are in
the attached archive Some more pertinent documents are in
the attached archive 
Attachment: SteamDistilationDocuments.zip (1.9MB)
This file has been downloaded 557 times
[Edited on 5-6-2014 by leu]
[Edited on 5-6-2014 by leu]
[Edited on 5-6-2014 by leu]
Chemistry is our Covalent Bond
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
More articles are in the attached archive 
Attachment: SteamDistillationArticles.zip (1.2MB)
This file has been downloaded 499 times
Chemistry is our Covalent Bond
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Wow. Don't know who to begin shooting Hi weeksie98, yes you are right and
probably reach a 1000 before I can pass the glassware exam. Hi weeksie98, yes you are right and
probably reach a 1000 before I can pass the glassware exam.
Hi Leu much of the discussion did not apply plus I read about the coffee machine. All I wanted was to know how safe this was, and since this is a
modification of what you can buy for 200 and 500 euros I needed to double check that I had not perhaps done something clearly wrong, (I read about
distillation apparatus exploding due to closed systems and so forth though I purposefully left this open, but I also read about release valves right
where the water is heating) since I have no idea about pressures and what could happen I needed to ask, I need to confirm with people on the forum
who have the benefit of experience since much of this particular information comes from hobbyists and not chemists, who, most of the time are dealing
with bought equipment and have a booklet. And I have never heard of the attached archive?
Hi Mallinmy, what do you mean by the trap, exactly where would that be? Should not matter since I will use de-ionized water anyway. And no, when tap
water is steamed it is not necessarily pure.
Sparkgap, yes that was the original plan, but unfortunately everywhere I read clearly stated that Lavender is one of those plants that should not be
placed into water, steam distillation for this plant, not water distillation, this got complex, so I can not repeat the reasons even though I read
them.
I will open that PDF and see what it is about, thankyou.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
A trap is a vessel between the steam generator and the flask holding the material of interest, with a tube going in, one going out. This is to catch
any drops of condensation that are coming over from the generator that formed in the tubing as to prevent them from going into a reaction mixture.
This is usually used in organic chemistry when only steam is desired and drops of water coming over into the reaction may complicate things. Don't
worry about having one for lavender distillation.
Why does lavender not fare well in boiling water though? The temperatures are he same and the oil will go into the vapor either way, no? Might be
different with lavender but whenever I steam distill plant material it is always from a mix of water and plant substance, topping up the water after
every 30ml of distillate is collected and the distillate is no longer cloudy (indicating oil/water emulsion)
[Edited on 5-6-2014 by Mailinmypocket]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
These are the references I kept, but I can assure you that I read some things this morning that talked about Lavender being particularly sensitive in
boiling water with reasons that went above my head, for example I want to do calendular as well but this leaf and plant can be done in boiling water
without breaking whatever can be broken, sorry I know next to nothing at this moment, just trying to learn yet another skill.
If extended exposure to hot water is not indicated for a particular plant - such as lavender, it is best to find an extraction method better suited.
Any botanical material that contains high amounts of esters do not take well to this extraction method, since the extended exposure to hot water will
start to break down the esters to the resultant alcohols and carboxylic acids. http://www.essentialoils.co.za/water-distillation.htm
Attempting to distill these Essential Oils directly from the plant material is generally not feasible. In general, most of the Oils' constituents are
high boiling and will decompose under the high heat needed to bring them to a boil. Steam distillation is a much gentler method of achieving the same
end. http://infohost.nmt.edu/~jaltig/SteamDistill.pdf
Steam distillation is used in the extraction of Essential Oil from the plant material. It is a special type of distillation or a separation process
for temperature sensitive materials like oils, resins, hydrocarbons, etc. which are insoluble in water and may decompose at their boiling point. The
fundamental nature of steam distillation is that it enables a compound or mixture of compounds to be distilled at a temperature substantially below
that of the boiling point(s) of the individual constituent(s). Essential Oil contains components with boiling points up to 200°C or higher
temperatures. In the presence of steam or boiling water, however, these substances are volatilized at a temperature close to 100°C, at atmospheric
pressure. [2] http://ethesis.nitrkl.ac.in/1949/1/satish_final_thesis.pdf
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
I'm still not sold on the claim that the ester components of lavender oil will decompose in hot water; if the components listed here are any indication, a cursory lookup of properties in handbooks do show that these ester components are high-boiling (hence the need for
steam-distilling in the first place), but no indication that they decompose.
This now suggests an opportunity for an experiment, if you're up for it: do two distillation runs, one with "internal" steam, and one with "external"
steam. Compare then, through appropriate means, the results from the two runs. Maybe even a qualitative test would suffice: if both smell just about
the same, then the claim about the unsuitability of "internal" steam is bunk.
sparky (~_~)
[Edited on 6-6-2014 by sparkgap]
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
Page 175 of Volume I of Guenther's The Essential Oils is attached 

Chemistry is our Covalent Bond
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
leu, thanks for posting that! So it seems linalyl acetate is indeed hydrolyzed rather easily by boiling water. Most peculiar...(That is, I wonder
what's so special about this ester that it's sensitive to boiling water?)
Fortunately, the solution is also mentioned in that page: push the steam through the flower spikes and into the condenser. This is easy if you have a
steam tap on hand, but if not, maybe have something like a column partly stuffed with glass wool above the boiling water, with the flower spikes
sitting on top of the glass wool.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
I am going to use a plastic sieve cut up to size, suspended inches from the base of flask. Flowers and leaves on top. Then the inlet pipe bringing
the steam to bottom of flask, but then find a way to disperse this steam so that it is not concentrated into one spot. Maybe attach a flexi hose with
holes all over it but blocked at the opening end so that the steam is then forced through those minature holes. Just thought I would share my
thoughts. Sorry about not being more professionally literate about references, it was hard finding anything with scientific details that would
satisfy the interrogators among you.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
From:
http://www.sciencemadness.org/talk/viewthread.php?tid=5950&a...
which was linked to recently:
For Rapid Steam Distillation Laboratory steam distillation is often a slow process if the compounds concerned have vapor
pressures lower than that of aniline. Increase in speed can be attained by increasing the temperature through superheating, increasing the rate of
steam flow, or by securing a more nearly perfect equilibrium between the steam and the organic vapor. The open tube often used as a steam inlet in
laboratory work has the effect of bypassing a considerable portion of the steam. Introduction of steam through a number of smaller orifices is an
obvious improvement. A steam inlet made of 1/4" copper tubing is often more convenient and more satisfactory than one made of glass. Eight or ten
slots are cut near the lower end with a hacksaw, and a 1/16" hole is drilled at a point that will be just above the liquid surface in the distilling
flask. The slots admit steam in thin jets, the hole permits equalization of pressure when the steam is cut off so fluid is not sucked back into the
steam line or generator. SAE tube fittings are much superior to rubber tubing for connecting such a steam inlet the supply line. The common
superheater, made from a coil of copper tubing, may be made considerably more efficient by winding it in the form indicated below. The several turns
are held in position by interlacing stays of iron wire. A cone shaped sheet of iron in the center spreads the flame, and the iron cover forces all of
hot gases to flow out between the coils. The outer case is open at the top.
with the illustration of the superheater now attached 
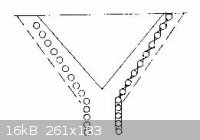
Chemistry is our Covalent Bond
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
I'll be honest Leu, despite the trouble you take to post this, I can not visualize where on earth this drawing fits in to the whole picture, the
description above leaves me confused around the point of 1/16th inch hole sentence. A simple straightforward drawing would save a million words. And
as for the other thread which for the second time I tried to go through, for me it's like reading they are building a multi industrial complex. I am
interested in dispersing the steam below the organic content, safety and making sure the system is optimized as best as is practical in a home/small
lab environment. I do not understand why copper is preferred over glass, except that copper disperses heat very efficiently, has this something to do
with it? If using a copper pipe from the steam inlet to the steam output is more efficient for some reason then great - I can adapt - no shortage of
copper here.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
That copper tube is used since slots can be cut into it for the steam to vent which allows more steam to enter the solution in the example  In your case you would probably want to use a metal sieve to hold the lavender, and
a copper tube wound in a spiral underneath with progressively larger slots cut into the top of the copper tubing so as to equalize the pressure In your case you would probably want to use a metal sieve to hold the lavender, and
a copper tube wound in a spiral underneath with progressively larger slots cut into the top of the copper tubing so as to equalize the pressure  The superheater should make the extraction of the esters in lavender more efficient
as well The superheater should make the extraction of the esters in lavender more efficient
as well 
Chemistry is our Covalent Bond
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
This all seems like hard-going for a relatively simple process.
CHRIS25 just wants to steam distill some lavender !
1. Drink 2 cans of beer. Soft drinks are optional, but not recommended 
2. Twist off the ring-pulls, saving as they are Al
3. punch a lot of small holes (hammer, nail, repeat) in the Bottom of one can.
4. partially fill the Intact can with water
5. Gaffer (Duct) tape the can with the holes on top of the can now holding the water.
6. Fill the top can with what you want to steam distill.
6. hot-melt glue some plastic or rubber tubing into the hole of the top can.
7. Attach tube to condenser
8. Heat the stack, start the water pump, wait for distillate.
Optional : poke a hole, then a thermometer into the top can, hot-melt-glue in place to retain seal.
Simples.
UPDATE: i just stuffed a handful of wild rosemary into an empty 330ml beer can, and it was 15g, with plenty room for more.
I will now drink a lot more beer to obtain raw material (empty cans).
The things we do for science ....
[Edited on 6-6-2014 by aga]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Aga@ I like your down to earth apparatus and for this I would wear a kitchen apron proudly, but my ambition is to one day put on that lovely white
coat and proudly be able to name all that glassware........
Leu@ ah now that makes sense, though those progressively larger slots at the "top" I presume you mean closest to the organics?
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Canware.
Heineken Steam Evaporation Cylinder
Skol 330ml Steam Dispersal Reaction Chamber
You can get a 500ml Stella version of each, but it is rather more expensive.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Quote: Originally posted by aga  |
I will now drink a lot more beer to obtain raw material (empty cans).
The things we do for science ....
[Edited on 6-6-2014 by aga] |
^^^ROFL
24 oz can=709ml. And the "apparatus" is a great suggestion, actually, as I would like to do some steam essential oil extractions. (poor fuck here)
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Quote: Originally posted by aga  |
1. Drink 2 cans of beer. Soft drinks are optional, but not recommended 
2. Twist off the ring-pulls, saving as they are Al
3. punch a lot of small holes (hammer, nail, repeat) in the Bottom of one can.
4. partially fill the Intact can with water
5. Gaffer (Duct) tape the can with the holes on top of the can now holding the water.
6. Fill the top can with what you want to steam distill.
6. hot-melt glue some plastic or rubber tubing into the hole of the top can.
7. Attach tube to condenser
8. Heat the stack, start the water pump, wait for distillate.
Optional : poke a hole, then a thermometer into the top can, hot-melt-glue in place to retain seal. |
As a further modification, you can puncture another hole on the side of the water-bearing can, then attach a tube connected to some convenient water
source, so that you have a convenient inlet for water if the previously introduced water has all steamed out. Certainly beats having to disassemble
the whole shebang should you run fresh out of water mid-distilling.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Beer cans to small, but, minature beer barrels, thicker, and definitely the right size.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by CHRIS25  | | for this I would wear a kitchen apron proudly, but my ambition is to one day put on that lovely white coat |
Doesn't matter what you're wearing, the Chemistry remains the same.
The main difference Chemistry can make to what you wear is the eventual requirement to put your shirts on backwards, so the straps can be tightened.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Just to test the theory, a "Candenser"tm got built today.
Basically it is a steam distillation rig built totally from tin cans, inlcuidng the condenser and receiver.
The boiler is just a tin can. Beneath it is a cut-down tin can which supports the boiler and houses tea-light candles.
The can above it has holes punched in the bottom, and contains whatever you are steam distilling.
The tube from there to the condenser is another can, the top and bottom cut off, then rolled into a tube just small enough to fit into the can
opening. On releasing it, the spring action makes a prety snug fit, and some mud was also used.
Two sticks were used to bend it at about 90 degrees, then released slightly to prevent blocking the tube.
The condenser is another can with one hole punched in the bottom. Around it are 6 more cans, taped together with duct tape. These are filled with cold
water.
Due to the weight, on each side are two more tin cans also filled with water, simply to support the "Candenser"tm. All taped together.
The 'receiver' is another can cut in half.
All in all it worked, giving 5 mls rosemary smeling stuff, however it needs at least twice the number of candles, or a better heat source altogether.
Attached is the vapour temperature profile with notes.
  
Attachment: candenser.xls (17kB)
This file has been downloaded 512 times
[Edited on 10-6-2014 by aga]
|
|
|