| Pages:
1
2 |
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Very useful infomations,you can use H2O2 inatead of PbO2
Isn't it air sensitive?amazing that you obtained the complex in presence of air and light
I go for cobalt salt and making the complex,now I don't have enough ccobalt salt
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
I don't think it's air sensitive, as the complex is formed in the presence of an oxidizing agent. The cobalt is already in the +3 oxidation state, so
I don't imagine oxygen from the air will oxidize it to the +4 state under normal conditions.
The ferrioxalate complex is also light sensitive, but I guess these complexes are light stable enough to be isolated at least.
I've made other Co (III) complexes with H2O2, so I'll try the synthesis with H2O2 instead of PbO2 as per your suggestion. Hopefully I'll have some
results to post about tomorrow
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Justin Cobalt is already in +2 state not +3.for it hydroxides ,oxidizes to +3 and for this complex reduced to +2.I will use your procedure to make
this.thank you very much
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
I meant the Co+3 in the complex is unlikely to undergo oxidation by oxygen in the air under normal conditions.
I attempted the procedure for K3[Co(C2O4)3]-3H2O on 1/10th the scale in the paper. I replaced
the PbO2 with 20 ml of 17% H2O2. The reaction proceeded as outlined in the paper until I added 50 ml denatured
ethanol, which did not cause the product to precipitate. I thought this might be due to the added water from the peroxide, so I added an additional
20 ml of alcohol. This did not cause precipitation of the product either, so I am chilling it in a freezer overnight.
The synthesis appears to proceed with lead (IV) oxide replaced with hydrogen peroxide, but it does make the isolation a little more challenging due to
the additional solvent.
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
For trisoxalato chromate,I have the same problem,it did't precipitate,I dont know why,even in addition of alcohol and freezing
I think it would b better to use PbO2,your water of hydrogen peroxide cause the disolution of complex,.maybe MnO2 would work,if you dont have PbO2
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
Luckily, that same document I posted has a procedure for PbO2, so this will be my next endeavour.
Update on the current reaction; a light colored (maybe white, but it's hard to tell because of how deeply green the solution is) solid has
precipitated. My guess is potassium oxalate, due to its limited solubility in alcohols. No sign of green crystals yet.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This link has a description of how to make oxalatochromate(III) complexes. I used these methods. They are based on reduction of dichromate to
chromium(III) in the presence of oxalic acid.
http://wwwchem.uwimona.edu.jm/lab_manuals/c10expt15.html
|
|
|
rumunpl1995
Harmless

Posts: 6
Registered: 3-7-2014
Location: Cracow/Poland
Member Is Offline
Mood: No Mood
|
|
Hey guys;
Recently I have done a project all around oxalate complexes. If you were interested I could post pictures of Mn(III),Cr(III)(trisoxalate +
cis-dioxalate + trans-dioxalate),Cu(II)(dihydrate),Co(III),Fe(III) and their absorption graphs (in water and in solid state). My team including our
chemistry teacher put a lot of effort in this project. We`ve also managed to prepare a poster but it is in Polish (If ya were interested I can
translate !!!!) .
When it comes to Co(III) complex I ve managed only to make it with PbO2 (Pb3O4 also worked but despite stechiometric amounts yield was pretty bad  ). Peroxide worked slightly I could see a green tint for a few seconds but after a
while all Co(III) was reduced to (II). I ve done this complex like twenty times since first synthesis. If you want to get high yield you have to do it
as quick as plausiable (the best way is to chill solution in fridge for like 15-30 mins and then filtrate). ). Peroxide worked slightly I could see a green tint for a few seconds but after a
while all Co(III) was reduced to (II). I ve done this complex like twenty times since first synthesis. If you want to get high yield you have to do it
as quick as plausiable (the best way is to chill solution in fridge for like 15-30 mins and then filtrate).
And by the way I am a big FAN of YOUR SITE WOELEN. THANKS for such a magnificant work !!!!!!!!! 
Waiting for replies. 
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
I'd be very interested in seeing your work. I actually just finished making lead (II) acetate, for conversion to PbO2, in pursuit of the Co (III)
complex.
|
|
|
rumunpl1995
Harmless

Posts: 6
Registered: 3-7-2014
Location: Cracow/Poland
Member Is Offline
Mood: No Mood
|
|
@sasan
Co(III) complex is not air sensitive it decomposes water (to oxygen) (like Mn(III) ) while reducing to +II oxidation state but it is light sensitive
and should be stored in amber vials.
@Justin Blaise
I`ll keep my fingers crossed for you.  (dont be suprised if your solution will be
very intense dark green in colour almost black (dont be suprised if your solution will be
very intense dark green in colour almost black  ) )
Pictures: (31 MB PDF file may take about 5 min to download )
<a href="http://speedy.sh/uusNF/Ox-pictures.pdf">Ox_pictures</a>
Stay tuned , more goodies are coming 
[Edited on 4-7-2014 by rumunpl1995]
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Maybe you are right rumunp.thank you for pixs.I will post pictures of potassium bisoxalato chromate and trisoxalatochromate I have made.
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
   
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Top left is potassium bisoxalatochromate(lll) it has a dark and very shiny green color comparing to the dull color of trisoxalatochromate
top right is potassium bisoxalatocuprate(ll) one of the most beautiful compounds I've made
bottom left is crystaline potassium trisoxalatochrmtae(lll) it is has dark dull green color
bottom right is pulverized potassium tisoxalatochromate(lll) green under sunlight blue under fluorescent lights
|
|
|
rumunpl1995
Harmless

Posts: 6
Registered: 3-7-2014
Location: Cracow/Poland
Member Is Offline
Mood: No Mood
|
|
Absorbtion spectrum (300-800nm) for trisoxalate compunds in solution:
<a href="http://speedy.sh/jj9vd/Ox-chart-solution.pdf">Ox_chart_solution</a>
Spectrum in solid state will be post soon  (are you interesed in seeing more or
less identical graph or should I use raw reflectance data instead?!?!) (are you interesed in seeing more or
less identical graph or should I use raw reflectance data instead?!?!)
@sasan
Make Co(ox)3 this compund crystalizes in amazing star alike structures 
[Edited on 4-7-2014 by rumunpl1995]
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
@rumunpl1995 , those pictures are beautiful. Nice graph, as well. Thanks for sharing! I would be interested in any additional
information you'd like to share; even if it is a nearly identical graph.
Would you be willing to share your procedure for the Mn complex?
|
|
|
rumunpl1995
Harmless

Posts: 6
Registered: 3-7-2014
Location: Cracow/Poland
Member Is Offline
Mood: No Mood
|
|
@Justin Blaise
K3[Mn(ox)3]*3H2O synthesis (our modified method) :
5 grams of H2C2O4*2H2O was dissolved in 35 ml of water in a beaker placed on a magnetic stirrer equipped with hot plate and warmed up to 65 C
(temperature can not raise above 75 C ) 1 gram of KMnO4 was very carefully added to the reaction mixture (in very small portions , check your
temperature frequently , you must keep your temps below 75 C). White precipitate appeared, solution during stirring changed color. After complete
addition of KMnO4 reaction mixture was let to stir until it was fully mixed and completely clear. 1,1 grams of K2CO3 was added in portions , the heat
was turned off, beaker with solution was placed in an ice bath and cooled to 20C - 30C after that 25 grams of crushed ice was further added (to the
ice bath I think so  ) and when reaction mixture was 2C 0,24 grams of KMnO4 was
added. Solution turned red and was passed through Büchner funnel with a sintered glass disc. Eluate was collected and treated with 35 ml of ice
cooled ethanol and placed in freezer. After 45 minutes when no precipitate was observed excess of ice cold ethanol was added until the precipitate
crystalize out. Precipitate was obtained by filtration through Büchner funnel with a sintered glass disc flushed earlier with a couple HCl drops.
Precipitate was flushed with cold ethanol then cold acetone and was left to dry in a DARK place. ) and when reaction mixture was 2C 0,24 grams of KMnO4 was
added. Solution turned red and was passed through Büchner funnel with a sintered glass disc. Eluate was collected and treated with 35 ml of ice
cooled ethanol and placed in freezer. After 45 minutes when no precipitate was observed excess of ice cold ethanol was added until the precipitate
crystalize out. Precipitate was obtained by filtration through Büchner funnel with a sintered glass disc flushed earlier with a couple HCl drops.
Precipitate was flushed with cold ethanol then cold acetone and was left to dry in a DARK place.

[Edited on 4-7-2014 by rumunpl1995]
|
|
|
rumunpl1995
Harmless

Posts: 6
Registered: 3-7-2014
Location: Cracow/Poland
Member Is Offline
Mood: No Mood
|
|
Absorption spectrum (200 - 900nm) for oxalate complexes Mn(III),Co(III),Fe(III),Cr(III),Cu(II),Ni(II) in solid state:
<a href="http://speedy.sh/Fpqg2/Ox-chart-solid-state.pdf">Ox_chart_solid_state</a>
Note: Ni(II) complex (light blue powder) did not disolved in water (Cu(II) also "decomposed" after 2-5 minutes depending on concentration, Ni(II)
complex didnt disolved at all), maybe thats only [Ni(H2O)4]C2O4 or [Ni(NH3)4]SO4 (adding ammonia was part of procedure) ??
(I really do not know why it didnt disolved at all and why copper complex decomposed on contact with water after couple minutes)
Any proposals??
That`s the last piece of data. 
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
Thanks for that procedure rumunpl1995! There appears to be a little more going on in the solid state spectra.
I followed your procedure and was able to achieve about 60% yield of the Mn complex on the left. On the right is the Cu complex. Still working on
the Co (III) complex.

Additionally, I was heating the Co (II) complex in a test tube to see if it produced Co metal (like Fe (II) oxalate) or the oxide and observed some
thermochromism from it. It was pink at room temperature and turned purple when lightly heated with a flame. On cooling, it reverted back to pink.
I was able to repeat the color change 3 times before the compound decomposed into a black powder.
 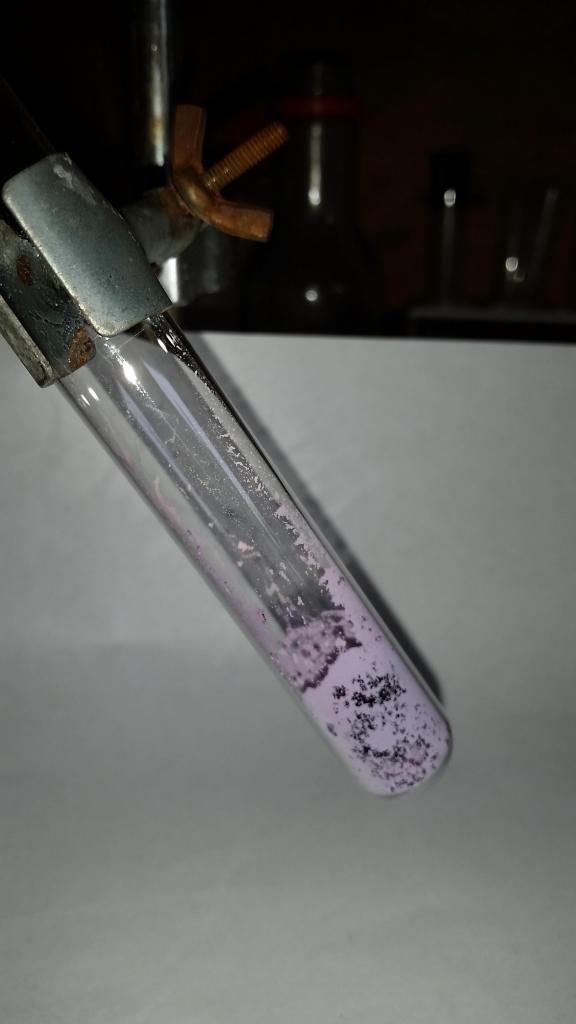
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I've got a good yield of K3[Co(C2O4)3] by the method from "Inorganic syntheses" (0.5 scale). While the main batch is being dried in a dark place (also
I used a red photo light at the last stages) I can show the part which I've got adhering to the funnel. I will use it to check the light sensitivity
(and will compare it with the crystals from the main batch in a few days).
By the way, which method could you propose to measure/compare the light sensitivity?
 
Comment on the photos: the color reproduction of my (phone) camera is incorrect here. The color is emerald-green/uniform. I believe the effect is due
to high reflection ability of the crystals.
[Edited on 8-1-2022 by teodor]
It should be mentioned that the water solubility of the complex is very high, for this reason, I performed washing with ethanol/methanol.
[Edited on 8-1-2022 by teodor]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I have had some luck making similar complexes with malonates, but I found with the iron(III) complex, I had contamination with potassium malonate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
DraconicAcid interesting, I will try to find a way to try it with malonic acid.
What I know at the present moment:
1. the formula is K3[Co(C2O4)3] * 3.5 H2O. I didn't find any publication whith the structure investigation (yet). Some publications which I found say
that the water is "probably coordinated". It would be interesting to know the actual structure, especially how water molecules are distributed.
2. after 2 days of keeping the Petry dish under direct sunlight there are visible things of decomposition on the crystal surface and edges (clearly
visible under a microscope).
3. I didn't find yet any other solvent apart from water for this complex. Question 1 in this regard is interesting.
4. Observing the crystals under a microscope reveals 3 types of them - green, blue and bluish-green. Bluish-green is the most abundant color but it
often has a green form as inclusion (like 2 sides of the same crystal can have a different color). Also, green and blue form forms separate crystals,
also blue crystals are different in shape. I think the blue form is a result of impurities (Nickel?) and the green form is a complex isomer.
Now the most interesting questions for me are 1 (structure) and 3 (alternative solvent).
Update: Methanolic HCl works as a solvent giving the same color of the solution as water (green) but after some time it changes to Co(II) color
(blue). When the water is added drop by drop, the color remains blue but at some point, it changes from blue to almost clear but slightly
grayish-green in one step.
The reduction of Co(III) to Co(II) doesn't go at the same speed in water HCl as methanolic HCl (hours vs 1-3 minutes)
[Edited on 11-1-2022 by teodor]
[Edited on 11-1-2022 by teodor]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
OK, summarizing my experiments with K3[Co(C2O4)3] * 3.5 H2O.
There are a lot of articles studying this compound. The best one which answers most of my questions is:
"Copestake T. B. and Uri N. 1955. The photochemistry of complex ions: photochemical and thermal decomposition of the trioxalatocobaltate III complex.
Proc. R. Soc. Lond. A228252–263
http://doi.org/10.1098/rspa.1955.0047"
So, reducing Co(III) to Co(II) in methanolic HCl is not because of the action of Co(III) upon methanol but due to thermal decomposition of the complex
which is speeded up by H+ concentration and presence of an organic substance. But in all the cases Co(III) acts as an oxidizer on oxalic acid only.
The mechanism by which the thermal decomposition rate is increasing with the presence of the organic substance is not clear. In my experiment, even
diethyl ether (which doesn't dissolve the complex even in the presence of HCl and always forms a top clear level) causes almost instant reduction to
Co(II).
The properties of "organic substances" which increase decomposition rate are unknown.
And the structure of the complex is really very complex. The anion is 2 Co(C2O4)3 centers bound together by some interaction of oxalic acid ligands.
The position of water is unknown/unclear. In my experiments, mixing with acetyl chloride doesn't change the crystals.
[Edited on 12-1-2022 by teodor]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
For the formation of the copper amine oxalate, I suggest trying the action of H2C2O4 on the base copper amine hydroxide. I have prepared copper amine
sulfate from the action of this base on aqueous MgSO4. In the current context, just use H2C2O4 for copper amine oxalate and water.
The copper amine hydroxide can be created in a mixed standard and electrochemical reaction with copper, pumped in oxygen (or some starting H2O2
followed by aeration as I am not sure if residual H2O2 will decompose the eventually created oxalate salt) along with dilute aqueous ammonia, and for
an electrolyte, a touch of NaCl.
General reference on the associated chemistry, see https://www.academia.edu/292096/Kinetics_and_Mechanism_of_Co...
Note, with a fall in pH associated with the consumption of ammonia, a problematic side reaction with the formation of some NH4NO2. The latter can
decompose at less alkali pH to liberate suddenly large volumes of N2 gas. This means use an open large vessel and be mindful of a possible spillage
event.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
For the formation of the copper amine oxalate, I suggest trying the action of H2C2O4 on the base copper amine hydroxide. I have prepared copper amine
sulfate (and nitrate) from the action of this base on aqueous MgSO4 (and Mg(NO3)2). In the current context, just use H2C2O4 for copper amine oxalate
and water.
The copper amine hydroxide can be created in a mixed standard and electrochemical reaction with a small solid piece of copper metal, pumped in oxygen
(or some starting H2O2 followed by aeration as I am not sure if residual H2O2 will decompose the eventually created oxalate salt) along with dilute
aqueous ammonia, and for an electrolyte, a touch of NaCl. Note: this is a spontaneous electrochemical cell reaction, one does not have to attach any
wires.
General reference on the associated chemistry, see https://www.academia.edu/292096/Kinetics_and_Mechanism_of_Co... which is employed in the commercial dissolution of copper metal ore.
Note, with a fall in pH associated with the consumption of ammonia, a problematic side reaction with the formation of some NH4NO2. The latter can
decompose at less alkali pH to liberate suddenly large volumes of N2 gas. This means use an open large vessel and be mindful of a possible spillage
event.
[Edited on 13-1-2022 by AJKOER]
|
|
|
| Pages:
1
2 |