| Pages:
1
2
3 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Zyklonb  | I added 15.4 grams of GABA (.15 mols) with 10.4 grams of NaNO3 (.15 mols). This was dissolved in 34.8 mL of water. I then added .16-17
mols of HCl (aq) drop-wise over a period of three hours.
So now I have a clear solution of GHB with sodium ions chloride ions and probably a bunch of other stuff. |
What makes you believe that you have a "solution of GHB with sodium ions chloride ions and probably a bunch of other stuff"? Do you have any analyses?
I'm pretty sure you have nothing but the starting materials there given that you substituted sodium nitrite for sodium nitrate.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Crap, my mistake. I did use nitrite, not nitrate. That was a typo.
I looked for the actual equation, but couldn't find it, so I'm not sure what other "stuff" might be in there, if any. All I know is there are sodium
ions (from NaNO2) chloride ions (from HCl), and NO was given off.
Sorry, I don't know much about organic chemistry, this is one if my first experiments...
[Edited on 15-5-2014 by Zyklonb]
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Ok, well I decided to go ahead and distill it. We'll see how yields go. I guess I should have made a bigger batch, cause I have 500 grams of GABA and
2 lbs of sodium nitrite and lots of hydrochloric acid.
|
|
|
hive3
Harmless

Posts: 27
Registered: 3-7-2013
Member Is Offline
Mood: No Mood
|
|
Distillation
The extraction process is cumbersome and requires large amounts of solvents and pulls over more impurities in my experience. Using steam distillation
is much more effective, greener and leads to a much clearer product. See Below:
Synthesis with steam Distillation Purification.
Suggested experimental:
103.1g GABA and 69.0g NaNO2 is added to 1L of water. Stirring is started. 116.7g of 31.25% HCl is slowly added. pH is checked to make sure it's around
4-5 (if not add more HCl). Reaction is run 24hrs with no heating.
The expected yield is 86.1g of GBL. The whole mixture can be distilled to dryness, then 84.0g of NaHCO3 is added to the distillate, refluxed for 30
minutes and boiled down to a reasonable volume and enjoyed responsibly.
>Does GBL steam distill?
Yes, 1 part GBL and 9 parts water seems to do it alright... which is EXACTLY what's called for in the reaction. Distill off the water/GBL and react
the distillate with a base (eg sodium bicarbonate which is NaHCO3 which is Baking Soda ).
It's a very efficient way of purifying everything...
If you acidify the solution slightly (pH 4-6), then all of the GHB will turn into GBL eventually in the distillation and steam distill.
|
|
|
I Like Dots
Hazard to Self
 
Posts: 69
Registered: 10-4-2013
Member Is Offline
Mood: frisky
|
|
Ive heard multiple comments on this, but is there any truth to this?
Im guessing the GABA cyclizes (under what conditions?), forming a secondary amine (probably 2-pyrrolidone?) which turns into N-nitroso-2-pyrrolidone.
Just for reference:
Primary Amines with nitrous acid
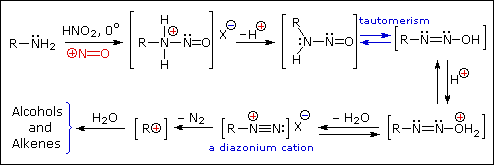
Secondary Amines with nitrous acid
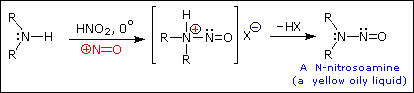
|
|
|
hive3
Harmless

Posts: 27
Registered: 3-7-2013
Member Is Offline
Mood: No Mood
|
|
Steam Distillation of GBL and Conversion to GBH
Quote: Originally posted by hive3  | The extraction process is cumbersome and requires large amounts of solvents and pulls over more impurities in my experience. Using steam distillation
is much more effective, greener and leads to a much clearer product. See Below:
Synthesis with steam Distillation Purification.
Suggested experimental:
103.1g GABA and 69.0g NaNO2 is added to 1L of water. Stirring is started. 116.7g of 31.25% HCl is slowly added. pH is checked to make sure it's around
4-5 (if not add more HCl). Reaction is run 24hrs with no heating.
The expected yield is 86.1g of GBL. The whole mixture can be distilled to dryness, then 84.0g of NaHCO3 is added to the distillate, refluxed for 30
minutes and boiled down to a reasonable volume and enjoyed responsibly.
>Does GBL steam distill?
Yes, 1 part GBL and 9 parts water seems to do it alright... which is EXACTLY what's called for in the reaction. Distill off the water/GBL and react
the distillate with a base (eg sodium bicarbonate which is NaHCO3 which is Baking Soda ).
It's a very efficient way of purifying everything...
If you acidify the solution slightly (pH 4-6), then all of the GHB will turn into GBL eventually in the distillation and steam distill.
|
I would like to point out that the stated 9-1 H2O to GBL ratio is not accurate in my previous post. The following procedure was followed for the
distillation and conversion to NaGHB:
On a hot plate with stirring the solution is distilled until salt starts coming out of solution and bumping starts. Approximately 800 ml of distillate
comes over. A second 800 ml of H2O is added to the solution and another 800ml of distillate is collected.
200ml of the 800ml first pass distillate is held in reserve. The PH of the distillate is 2.8. 30% NaOH solution is used to bring the ph of the
solution to 7.5. The 200ml held in reserve is to correct any overshooting of the PH past 7.5. 9.75g of NaOH neutralizes the first 600ml, and based on
this ratio, an additional 3.25g NaOH is added to the remaining 200ml of solution.
The resulting solution is boiled down to approximately 100 ml in a stainless steel pot on a gas burner. This solution is then placed in a small
aluminum pan and heated at 170C in a small toaster oven for 1.5 hours when bubbling stops. The pan is allowed to cool and 33.5g of NaGHB is collected
as a white soap like block in the shape of the pan. Previously removal of the H2O was attempted over the burner in the pot, but led to significant
discoloration of the final product due to uneven heating.
This process is repeated for the second distillation which requires 8.5g of NaOH and yields 26g of NaGBH.
Based on these recoveries, The first distillation recovered approximately 24ml of GBL and the second approximately 19g of GBL. Which is more like a
30:1 ratio of H2O to GBL.
Theoretical conversion of the GBL to GHB on the first run should result in 40g of GBH from 28g of GBL and 13g of NaOH. I was using NaOH that had
clumped to make my 30% solution so there was water in the NaOH that obviously skewed my results.
[Edited on 7-7-2014 by hive3]
|
|
|
infinite
Harmless

Posts: 4
Registered: 25-10-2014
Member Is Offline
Mood: No Mood
|
|
I want to synthetize Aceburic acid (4-acetyloxybutyric acid)
It is possible that in the reaction of sanmeyer, instead of using water, using glacial acetic acid this compound can form?
Maybe another route is to submit the purified anhydrous NaGHB in a glacial acetic acid reflux
Or maybe Acetic anhydride + butyrolactona , but i think that the yield of this, is very low.
Thanks for the answers
|
|
|
| Pages:
1
2
3 |