| Pages:
1
2 |
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Construction of oxyhydrogen blowpipe
Is it possible to make a simple oxyhydrogen blowpipe? The hydrogen and oxygen is to be supplied by electrolysis, but the trouble is to get it to burn
safely?
I need a little help here, the inthernet does not seem to document much about it, only that it is invented to melt metals( 2000degrees flame) and that
it is used to produce lime light in the ancient times.
The inventor used 2 sort of chambers to be filled with hydrogen and oxygen. Here I think that is not neccessary, electrolysis will do.
Anyone has an idea how to build one?
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
I've thought of building an oxyhydrogen flame torch too.
I wonder if 15 amps at 120 volts will give a nice sized flame?
Lets see- here's a quote from
http://www.h2cars.biz/artman/publish/article_467.shtml
~
The energy required to produce hydrogen by electrolysis is about 32.9 kW-hr/kg. A kilogram is about 2.2 lb. For 1 mole (2 g) of hydrogen the energy is
about 0.0660 kW-hr / mole. For commercial electrolysis systems that operate at about 1 A/cm2, a voltage of 1.75 V is required. This translates into
about 46.8 kW-hr / kg, which corresponds to an energy efficiency of 70%. Lowering the voltage for electrolysis, which will increase the energy
efficiency of the process, is an important area for research. Intense research persists to better understand ways to improve these hydrogen production
methods.
~
So at 66 watts-hour/mol, and if we get at most about 1700 watts of power from the oulet, that means every hour, about 20 moles of H (H2?) will be
produced.
Add about 10 moles of O, and I get 15 mols of H2 and O2. Maybe you get something different. Anyways, that's about 300 L of gas per hour, or 5
liters per minute.
Is that enough gas for a useful flame?
Now I probably unbelievably botched that, but anyways, you get the point, I'm not sure you'll get enough gas easily, and storing up gas for
later use in homemade tanks would be slightly unnerving. 
Of course you don't want the H2 and O2 to mix before they really need to, unless your flame is guaranteed to move faster than the burning rate of
H2 and O2. That would be a risky assumption.
I'm assuming a standard transformer and dc rectifier would work, but it would be quite a rectifier!
I have no clue what to use for affordable anodes/cathodes. Anyone? 
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
nickel anode/ stainless steel(304 or 316) cathode 30% KOH electrolyte.
Fellow molecular manipulator
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
Do a search for "water welder" or Brown's Gas. There are several overpriced systems out there that burn the premixed (!!!!!), generated
gas, sometimes enriching it by bubbling it through methyl alcohol, and using a hypodermic needle as a torch tip. The manufacturers claim it is even
hotter than the stochiometric mix, alluding to some atomic hydrogen floating around in the mixture. It is supposed to weld platinum. They used KOH
and water as an electrolyte. It looked easy enough to build, maybe even using an old PC power supply.
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Thanks for the info. Methinks that premixing H2 and O2 and then lighting one end sounds dangerous, but if the Official people do it, then it must be
ok. 
I don't think a comp. power supply is going to cut it- they put out, what, 35 amps or so? At 1.5 volts, to get 5L of gas per hour, we're
talking about 1100 amps! 
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
http://www.dangerouslaboratories.org/hydrogen1.html
The amps would have to be upped or the gas somehow pressurized if this was to be used as a cutting/wlding torch of some kind.
[Edited on 10-1-2005 by rogue chemist]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Plans for a 2H2O2 generator I found while researching the subject, looks a bit complicated though:
http://jlnlabs.imars.com/bingofuel/bgen/
Btw, does anyone know of a catalyst that will make an H2/O2 mix ignite spontaneously? Would a very small Pt fragment do it?
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
 Seriously, this looks complicated..I was thinking that the products of electroysis
being used straight away, by going thorugh a sort of compressor? I don't intend to store this mix, its dangerous. Another problem is to have
enough current to generate that gas mixture at a high enough rate. Seriously, this looks complicated..I was thinking that the products of electroysis
being used straight away, by going thorugh a sort of compressor? I don't intend to store this mix, its dangerous. Another problem is to have
enough current to generate that gas mixture at a high enough rate.
To make H2 O2 ignite spontaneously? I was thinking of using a bit of chlorine(mad idea)..You know, by dissolving just enough sodium chloride to
saturate the solution, you could get a mix of H2 O2 and Cl2. UV detonates the mixture spontaneously.
Edit: http://www.cci.ethz.ch/mainpic.html?expnum=23&ismovie=1&...
Look at this, although this has nothing to do with the blowpipe, but the way the hydrogen is generatee and ignited is interesting.. The flame seems to
be floating.. Someone needs to translate the page.
[Edited on 10-1-2005 by darkflame89]
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
mick
Hazard to Others
  
Posts: 338
Registered: 3-10-2003
Member Is Offline
Mood: No Mood
|
|
Btw, does anyone know of a catalyst that will make an H2/O2 mix ignite spontaneously? Would a very small Pt fragment do it?
Adding extra 5% Pd on carbon or 5% Pt on carbon to a reduction mixture saturated with hydrogen and organic solvent will usually ignite. You have to
purge the kit with N2 or Ar before adding extra catalyst. I think the activity depends on the surface area of the metal so small bits of the metal
might not work or it might need pre-heating. I seem to remember methanol or something similar and air can get Pt wire up to glowing heat. There used
to be a cigarette lighter that worked by sucking air through it and the wire coil glowed and lite the cigarette.
mickl
mick
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
NaOH or KOH ?
KOH as the electrolyte and Ni electrodes seem to be the textbook method for 2H2O2 electrolysis.
I'm curious as to if NaOH (being very much more available than KOH) could substitute for the KOH, and if so, what caveats apply? Is it only the
anode that needs to be Ni? Cathode as well? If not both, what can be used for cathode? All questions, no answers...
I'm going to build a small 600W 2H2O2 generator, and have found a source for nickel, but I'd much rather use NaOH than KOH.
I've tried googling for answers, but all I get is a shitload of links to overpriced commercial systems without any interesting technical
documentation whatsoever.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Twospoons
International Hazard
    
Posts: 1280
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
The issue of current is easily solved by connecting multiple cells in series. So intead of one cell running 1000A at 1.5V, use 10 cells in series
running 100A at 15V total, or 20 cells = 50A / 30V etc...
Plumbing gets a bit more complicated though.
I've seen stainless used for electrodes on some designs - high Ni alloy ?
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
I can't find the site anymore but here is a graphic of a 'water welder' gas generator. Note the step down transformer, diode,
electrolytic cell, and one way valve in the plastic line. My apology to whoever owns the picture.

|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
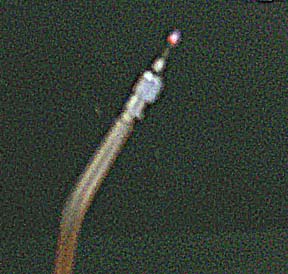
And here is the flame, at the end of a hypodermic needle. Also not my picture, I'd give credit if I could.
photo by:
I found the address in the user groups.
rec.crafts.metalworking
http://www.speff.com/ww1.jpg
[Edited on 19-1-2005 by Mr. Wizard]
|
|
|
uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
So we can build a cell, or several cells that have seperators over the anode and cathode to gather the 2 gasses independently.
Thats the easy part. The hard part is this. You wont be able to mix the two gasses and have a continuous flame unless you have a forced feed, and by
that I mean you need to maintain a certain flow. The flow needed will depend on the oriface where the gas will be burned, and the speed that the flame
moves. If your flow is too low, the flame will run back into a turbulant place in your pipe or as far back as you mixing chamber (called a flashback).
the flame will continue to burn up the gases thus far back and either choke eventualy, or create a well and run back into your H2 source with O2
running right behind it, spreading your equipment around the room with a nice orange ball of energy.
If your flow is too high, the flame will mip, (dance at the end of the gas with a gap between it and the oriface) or, it wont even light.
notes about hydrogen as a heating gas. when combusted with the right proportion of O2 (2H2 +O2) it could in theory produce an inner envelope that has
a temp of about 5300 F.(this is the gross, not the net) However... Hydrogen has a very low density, so its heating value for one cubic foot is very
low compared to other gas fuels that are made of larger molecules. H2 also has a higher flame speed than most other fuel gasses, so you must burn MORE
H2 to sustain your flame than any other gas fuel.
The smaller the oriface the better when it comes to H2/O2 otherwise you will have a hell of a time forcing hydrogen into your pipe at the rate needed
to sustain the flame.
I could go on and on about the different things you would need, (check valve, flashback arrestors, some way to regulate your endflows for mixing, shut
off valves, some way to force feed the gasses (put them under pressure), etc) The most important being a way to exactly regulate the mixing of H2 and
O2. you wont get your 3000 F flame if you dont get it exactly right. the changes are quite drastic when you make a carburizing flame vs neutral vs
oxidizing flames, and I imagine the difference in the gas mixture of H2 and O2 could be very small to produce the different mixtures. This may be the
reason a "professional" product would have had the gasses premixed since they are allready in the right proportion from 2H20 -> 2H2+O2,
and since you are reacting the opposite, hell thats great... and really hazardous.
but this method would not allow you to do any cutting, unless you used 2.89x of compressed air along with it, but then you get nasty nitrates and CO2
to deal with.
My thought would be... this is a cool idea, I mean having a cheap way to produce a hot flame that you could just bleed when your done. No storing
hazards or having to pay fees etc. But I really think that even if you could make a system work, it would not be safe. at all. you would not save any
money because you could weld 22-26 gauge steel with it.(haha)
You definatly would not be able to do much cutting with something like this, unless you were able to pump pure O2 down a 2nd oriface that wouldnt
react with the 1st oriface (so the 2nd path of O2 can go to react with the metal rather than the H2).
If you want something cheaper... try getting a butane, propane or MAPP canister. its cheap, its easy, its a hell of a lot safer. If you have more
money, *rent*(buying them costs a lot of cash. I think mine were $200 a pop) some gas cylinders and buy some acetylene and oxygen. Oxy/Acet is an
industry proven method of reaching 5600 F, and its been around for... a looong time.
on a last note, I have seen hydrogen gas cylinders hooked up to oxygen cylinders with oxy/acet torch equipment used... it does work, its just not very
practical without pressurized cylinders.
|
|
|
Oxydro
Hazard to Others
  
Posts: 152
Registered: 24-5-2004
Location: NS, Canada
Member Is Offline
Mood: distracted
|
|
I've tried something like this before, but I couldn't get the flame to sustain all that well. This site is the best resource I've found so far, even if the guy is into overunity and all that crap  . .
If you try it... try filling a 1L pop bottle with the mix and igniting it (remotely) You will find only fragments of the bottle. For something really
impressive, try the 5-gallon watercooler bottles. Althoug you need a lot of gas to do anything usefull, its explosive power if mistreated is rather
scary!
Make sure, if using mixed gas, to include a bubbler in your system (use water to isolate the storage from the flame). Make the space above the bubbler
as small as possible.... it only takes a few CCs to make an earsplitting bang and send the bubbler flying (the pop bottle I was using would compress
and then bounce off my desk).
My brother tells me that oxy-hydrogen flames are used for welding of small wires eg platinum electrodes -- he used to design paper-plant sensors for
Honeywell, and he contracted a jewler to use such a torch for delicate assemblies.
"Our interest's on the dangerous side of things" -- Browning
|
|
|
rift valley
Hazard to Others
  
Posts: 103
Registered: 24-7-2004
Location: NH
Member Is Offline
Mood: semiconducting
|
|
Wow thanks for the link, a lot of interesting stuff on that website, I have some questions though. Here is a picture of the cell he made it in
corporates 80 6"x6" #316 stainless steel plates (that couldn't have been cheap). The plates are spaced a few mm apart with
noncunducting plastic. The electrodes are attatched to each side, so there are 78 metal plates in there with no direct contact with an electrical
source. Is the reason for the extra plates to improve the conductivity of the cell? For some reason it is my instinct that one electrode should be in
direct contact with 40 of the plates and the other electrode in contact with the other 40, is it wrond to think this? Her is the picture I have been
referring too.

Before going to that link this thread was just a novelty, but now I am genuinely interested in pursuing this.
Thanks,
Rift Valley
P.S. Bubbleing the gases through water appears to be a fail safe way to prevent a flashback, or at least containing it to post-bubbler plumbing.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
I would think that each plate would get a voltage difference of a few volts between each one, and so when when a higher voltage is applied it is able
to be done at a much lower current, because the cell will be like 40 2V 10A cells, so the total current is 400A, even though the input is only 80V at
10A. That way the current is used as efficiently as possible.
At least that's what I would think happens. If so you could run the thing off rectified wall socket power, at 160VDC average. If you divided it
to have 2V between each plate, and had an input of 15A, then you would have an effective current of 1200A, without having to build a transformer that
can handle 1200A (not an easy task at all).
Assuming I'm right with my idea that is, but it seems like it should work.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Thread reboot
Based on pictures of an oxyhydrogen torch in this thread, I decided to do a little research on them. Here are some results.
Rio Grande (jeweler's supplier) sells what they call a "water torch" (category link), the Hydroflux Welder by Okai. Rio Grande sells NaOH for electrolyte; Okai sells KOH. The Rio Grande "Water Torch Flux Dry Mix" is boric acid. It's dissolved in methanol
to make a dilute solution. Electrolysis gas is bubbled through this; it picks it up as vapor. The effect is to make the flame slightly acidic and
slightly reducing, both good for jewelry work. There's a gas line filter for the unit with a sintered bronze filter tube. It's available as a fuel filter from JCWhitney. I'm sure this filter also acts as a flame arrestor; it seems like a generally good idea to use these sintered metal
filters for this purpose.
The tips on this burner are simple Luer Lock hypodermic needles. McMaster-Carr sells them under the category "dispensing needles". In this same category, though, they also sell heavier-wall needles that might work better mechanically. They've certainly got
more heat capacity and thus would cool a flame front better. McMaster-Carr also sells Luer Lock couplings to put on the torch head. @DougTheMapper: You can get easily switchable needles with this system, rather than hacking it
together with a barb fitting and heat-shrink.
From reading a discussion board something (sorry, reference lost), the Hydroflux operates at 3 psi gas pressure with some kind of manostat that
controls average electrolysis current. There's another brand of jeweler's torch with adjustable output.
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
I have decided to repost the start of my BYMC thread here since the topic at hand is a lot more relevant than the site where it was originally posted. Furthermore, I feel like I can
contribute useful material this way without constantly referring to another site. There was a bit of Q and A in the original thread that I'm not going to transfer, but I can always re-answer them here. Anyway, here's the post:
Hey all:
I just wanted to show you some photos of my oxyhydrogen torch project.


First off is the power supply. This is just my rewound microwave oven transformer to which a trio of parallel alternator diodes have been added.
As you can see, the diodes have been bolted to a large heatsink because they dissipate quite a bit of power while rectifying 15VAC to half-wave DC at
25A.

Here is a picture taken during the construction of the cell itself. All hardware is either 304-series stainless or nylon. 316L could be used for
longer-lasting plates, but that would make this cell significantly more than $10. Stainless steel is pretty hard to drill though because its chromium,
nickel, and manganese content make it very hard. I used a bit sharpener several times.

Here is the final assembly. The margarita bucket is the main cell and the two stainless steel rod terminals can be seen exiting the top. The vinyl
tubing leads from the cell to the bubbler which acts as a flashback arrestor since the oxyhydrogen mix is extremely explosive. The blue stopper is
just some rubber piece that happened to fit in the vent hole and should prevent total cell destruction should an explosion occur. It is also used in
conjunction with the tap at the bottom to swap the electrolyte. The bubbler is contained in a second bucket to catch the water released if it
explodes.

Here is a view into the cell after about a half hour of continuous operation at intermittent intervals over the course of an afternoon. The iron oxide
flocs in the electrolyte are expected and are a result of low-quality stainless steel. The plates show almost no stains or signs of wear and should
last for many hours of operation before they must be acid-etched to clean them. The electrolyte is a solution of NaHCO3 in water and has been adjusted
in concentration so the cell draws 25A when fully warmed.

The following photo shows the gas generator hooked up and in operation (note the bubbles present in the bubbler device and the distortion of the cell
lid from gas pressure).

Unfortunately, the following photograph is a bit blurry. It shows supply current and voltage drop across the cell. Admittedly, the battery in my
voltmeter is dying, so it reads a bit low. The cold cell is drawing 22.7A and dropping about 5V for a total power of circa 113W.

This is the torch itself consisting of some segments of half-inch galvanized iron pipe and various fittings and valves. The interior of the tube is
packed with very fine (#0000) steel wool. When a flashback occurs, the wool draws enough of the heat from the flame front to arrest it and prevent the
bubbler from exploding. This has been isolated and tested about 50 times with no breakthrough of the flame wave so I deem it a suitable safety device.

The following is a picture of the flame in a darkened room.

It may not look like anything significant, but you might be surprised. The incredible thing about this flame is that the two gases burn so fast that a
visible flame is only 8mm long - however, the hot exhaust of the flame carries much farther and will ignite paper over 4cm away, demonstrated here:

It's also easy to underestimate the heat this flame generates. Here, it has been trained on a 90/10 silica/bentonite refractory coupon. As you can
see, this torch has no problem vitrifying my 3000+°F refractory, something impossible to do with a even a propane torch. For reference, I'm burning
hydrogen in oxygen, which has an adiabatic (theoretical maximum) flame temperature of 5792°F. This tops an air/propane torch which maxes out at
3596°F and even rivals oxyacetylene at only ~400° cooler. Neat!

Anyway, the diodes tend to get hot fairly quickly so I have found a set of 5 power MOSFETs on a heat sink which should help to alleviate this problem
when configured to replace the diodes. I must also mention that the current torch made desoldering these MOSFETs and the heatsink an absolute dream.
Well, that's about the end of the latest lab developments. I hope you enjoyed!
-Doug
EDIT: Thanks for the info about the needle couplers. I'm still waiting for some money to open up for more robust rectifiers. I'm spending nearly $300
on glass and reagents for the holidays so I'm probably not going to get this project rolling until I actually have a use for it other than burning the
crap out of things.
[Edited on 14-12-2010 by DougTheMapper]
Victor Grignard is a methylated spirit.
|
|
|
crazedguy
Hazard to Others
  
Posts: 143
Registered: 12-11-2010
Member Is Offline
Mood: You can't fix stupid
|
|
This seems like a really good idea and very cool. Maybe try not lighting it fill a balloon tape fuse to balloon = KaBooom.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
An idea: To ignite H2 gas place it in contact with Cl2O (Dichlorine Mono-oxide) gas. Ignition to a mild explosion should result.
To prepare Cl2O: Pass a steady stream of air over concentrated HClO (Hypochlorous Acid) that has been saturated with Ca(NO3)2. This works as the
concentrated acid easily releases Cl2O (the anhydride of HClO) and Ca(NO3)2 is a drying agent.
Other methods: Pass Cl2 over heated NaOH. Or, Cl2, an inert gas and steam over heated Sodium carbonate.
Note: Due to it unstable and explosive nature with shock and light sensitivity, Cl2O should not be stored.
|
|
|
Master Triangle
Harmless

Posts: 14
Registered: 24-12-2013
Member Is Offline
Mood: No Mood
|
|
I am considering building an oxyhydrogen torch apparatus and am seeking feedback on the design.
The electrolysis cell would be constructed with about 50-60 plates so that I can use it directly from 120v from a stepdown transformer. I considered
building a cell or series of cells that you could apply rectified 240v across directly but I think that a little electrical isolation is in order,
what with all this electrolyte and possibility of explosions.
For the people who are still wondering about the stacks of unconnected plates in most cells they basically divide the voltage as the optimal
plate-to-plate voltage is somewhere around 2v and having a higher voltage just makes heat. There is a voltage gradient between the two end electrodes
and just putting an unconnected plate in between them will basically divide the cell into two cells of half the voltage in series, the current will
remain the same however so gas production is basically doubled.
One of my possible design ideas was an ultra-cheap method where you cut a thin stainless sheet into appropriate plate sizes, apply RTV or liquid
gasket around the edges, and sandwich together with some thicker end plates that have screws extending down outside the stack to hold it together. You
will have to avoid angled plates, if they are not parallel then there will be greater voltage and hence current flowing in the close area and the rest
of the plate area will be doing very little.
The cell voltage could be adjusted using a PWM controller before the rectification stage, some electrolytics would increase the efficiency a bit too.
The pressure would be maintained by having a pressure switch that turned on and off the cells to maintain a roughly steady pressure, or even better, a
PID controller.
Then have the usual flashback arrestors, sintered metal seems like the best option, I don't think I will put a bubble chamber in. The torch would be a
simple hypodermic which can be easily swapped out for various sizes, I will try to incorporate the flashback arrestor into the hand piece to reduce
the unprotected volume.
I am wondering about the burner design though, how wide can you make the nozzle before flashback occurs? Does it depend on gas velocity through the
nozzle? Can multiple nozzle be used aimed at the same spot? I don't think I will bother with any exotic methods of ignition, a lighter seems to work
well for most people.
Also the problem of spud guns using oxyhydrogen, it burns at about 1-2.5km/s producing a dangerously high shockwave and pressure peak, then the
pressure drops rapidly because the h2o just condenses. It is hard to get any work out of it because work=force*distance and it is hard to get anything
to go very far during the pressure peak. If anyone knows how this could be made to work (haha) better then that would be great.
|
|
|
TheChemiKid
Hazard to Others
  
Posts: 493
Registered: 5-8-2013
Location: ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by axehandle  | KOH as the electrolyte and Ni electrodes seem to be the textbook method for 2H2O2 electrolysis.
I'm curious as to if NaOH (being very much more available than KOH) could substitute for the KOH, and if so, what caveats apply? Is it only the anode
that needs to be Ni? Cathode as well? If not both, what can be used for cathode? All questions, no answers...
|
Sodium Hydroxide works almost as well.
When the police come
\( * O * )/ ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Might try to build one of these. Is a US nickel pure Ni, Plated Ni, or alloyed Ni?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
A US nickel is 25% Ni 75% Cu. There is a thread on extracting the nickel.
https://en.wikipedia.org/wiki/Nickel_(United_States_coin)
|
|
|
| Pages:
1
2 |