| Pages:
1
..
3
4
5
6
7
..
14 |
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Whats going on ?
ordenblitz, I used the 4-fold amount of benzoate and the 8-fold amount of sodium hydroxide thtas it.
Marvin will have known why he asked for scaled up results.
The problems are not only one:
- clinging of the fused mass to the glass threatening to destroy it.
and
- not all benzoate reacts. The fused mass is an nice insulator and makes it hard if not impossible to get all to react after a certain amount has
fused.
I am talking about 100g+ benzoate here, please realize that this is hardly comparable to 25g or less.
Yes. Scaling up is the interesting part and I believe I have an easy and cheap solution.
Later more.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Actually the reason I was hoping for scaled up experiments has nothing to do with chemistry. I just get a kick out of the idea something so
fundamentally important/useful can be made on a large scale from what is available.
I had an idea about protecting the glassware but Hermes beat me to it. Rather than use nickel though which I'm unsure if its easy to plate, use
silver. Silver metal is cheep in small amounts, reduce the salt with formalin say and precipitate onto the inside of a well washed with dilute NaOH
flask and that should produce a good layer of silver. Silver mirror test basically.
With some luck that will prevent destruction of the flasks.
When you are talking about 80 or 85C are you talking about the temperature of the vapour or the temp of the mass being heated. You arnt using a
reflux column are you?
Temps for the decomposition of sodium benzoate alone were in the 500C+ so I'm a little confused why the numbers given are so low, even if its the
vapour, why is it cooling down so quickly?
I will search around locally for some sodium benzoate but I wont be trying it for a while, I'm unhappy with my ventillation for anything
carcinogenic and as yet, I dont have any use for the benzene.
[Edited on 6-9-2004 by Marvin]
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
- The temperature named, 80-85°C is of course the temperature on the Claisen and measures the vapors coming over.
The temperatur in the flask is MUCH higher.
- Using a column would be not so good an idea. Liquid dropping back would rather probably crack the flask already during the reaction. (see the second
reference from S.C. Wack whoc was btw right that smoke indicates to high a temperature here and gives no more benzene).
- using a metal vessel or coating the glass with metal will solve perhaps the problem of the cracking, but wont solve the problem with the
heatdistribution when bigger amounts are processed.
I am optimistic to have found a solution for both problems now:
- cracking is no problem as disposable winebottles are used.
- heatdistribution is equalized by stuffing the bottle with steelwool, the cheapo kind used on wooden floors. (defat before use)
Looks VERY good! uiii!
Results and pictures will get posted after redistillation.
Results of the glassware-murder runs:
- first produced 25ml benzene from 100g benzoate and 60g NaOH. (flat bottom flask)
- second produced almost 35ml benzene. (Erlenmeyer)
So more NaOH gives better results. The Erlenmeyer gave better results what I blame on the more surface area.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Some images:
Here the bottle and the steelwool. I stuffed later more steelwool into the bottle, but one gets the idea I guess.

This is how I made the conection to the condensor (condensor still missing).
A cork, a 14 to 29 adapter and Teflon-tape. The adapter isnt essential, two corks and a metal tube (copper/steel...) and tape would do the trick to,
its not hard.

Results after workup. Looks good up to now.
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Organikum said:
----------------------------------------------------------------------------------------------------
not all benzoate reacts. The fused mass is an nice insulator and makes it hard if not impossible to get all to react after a certain amount has fused.
I am talking about 100g+ benzoate here, please realize that this is hardly
comparable to 25g or less.
----------------------------------------------------------------------------------------------------
Excellent point!
I was using a 500ml flask with 32 gm of reactants. That certainly would create enough surface area to get the job done more efficiently. Typically I
would add the mix, then spin the flask to make the powder ride up the sides before putting it in the mantle. And now that you mention it, I do
remember seeing the fused mass somewhat pulling away from the sides of the flask later in the process.
One think I didn't mention was that I heated fairly slowly. Usually it took 30 to 40 minutes before any condensate began to form. It is possible
that a gradual temperature rise allows for better heat transfer before the reaction progresses to fast in the outer layers thus insulating the mass
further inward.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Question for Marvin:
You wrote befor, that benzoic acid and conc. H2SO4 gives "decent" yields of benzene.
- What are "decent yields"?
- Wont this end with a sulfonation of the ring? Similar to toluene to toluenesulfonic acid?
- Or is this some equilibrium building up, say parts sulfonation, parts decarboxylation with benzene removed and the equilibrium shifts? As
sulfonation is reversibel as I know......
But would conc. H2SO4 be the right thing to use, wouldnt 70-80% be preferable?
A mechanism would be the hit.
merci
ORG
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Possibly similar to the dehydration of formic acid to carbon monoxide with concentrated sulfuric.
HCOOH ----> H2O + CO
I could see many different compounds forming from the dehydration of benzoic acid, benzene might be among them.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
And now: Whats that?

Thats what happens when you add NaOH (preferable aqueous, aka lye) to sodium benzoate dissolved in water.
This precipitate is what the author talks about in the second article posted by F.C.Whack.
Aha!
Sorry for the delays my coffee-grinder died the death of true heros - and I now can tell from own experience that finely divided sodium benzoate is
dangerous, ok, it was a "puff" and no "bumm" but it shows what can happen.
Ignited by the grinder, which seems to have died from shock. Or electrocution after the smell.....
Soon more, this precipitate looks even better to me...
Tomorrow to the second hand store for a new old grinder......
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Hmm. That sounds like something I'd say but I'm damned if I can find my post either here or on roguesci and Ive searched for an hour.
Which post did I say that in?
I dont think Ive ever been under the impression it would be a good method compaired to pyrolysis but I've heard other people mention it. I dont
have a description, but frogfot posted a general mechanism on roguesci in the phenole thread,
http://img37.photobucket.com/albums/v113/frogfot/stuff/decar...
Thats from March, but I think its a generic mechanism for decarboylation of an aryl carboxylate with sulphuric acid rather than a specific one for
benzoic acid.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
| Quote: |
Which post did I say that in?
|
Earlier in this thread. If you use "printable version" on top - left side, you get the whole thread in one piece and ctrl-f
"Marvin" will help you to find it then. 
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Actually, it was Turel who said that benzene can be produced from benzoic acid and H2SO4. It's a long and confusing thread by now, I know!
But he offered to explain things. So, Turel, how about the explanation for transformation of benzoic acid to benzene via H2SO4?
PGP Key and corresponding e-mail address
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Oh!
Yes it was Turel, I overread the name whilst skimming through the thread.
I apologize.
ORG
|
|
|
thefips
Harmless

Posts: 33
Registered: 6-3-2004
Location: Germany
Member Is Offline
Mood: self destructive 
|
|
I often think about the synthesis of benzene from phenol and Zn-dust.Has someone an idea how to make it?I want to make some experiments.I thought:
1.: heating an mixture of phenol and Zn-dust and looking,if tere is a distillate and the smell of benzene.
2.: heating phenol and passing the smoke over heated Zn-dust,I remembered that ethene could be made by passing EtOH-steam over heated Zn (or was it
Mg?I think it was Zn)
Which one could be possible or could be possible?I want to try it the next days.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Basic sodium benzoate ?
Marvin wrote:
| Quote: |
Basic calcium benzoate decomposes,
Ca.C6H5COO.OH => C6H6 + CaCO3.
|
Am I right when I guess that the precipitate formed on addition of lye to a solution of sodium benzoate is basic sodium benzoate? But what formula has
this?
calcium benzoate:
C14H10CaO4
C6H5COO-Ca-OOCH5C6
+Ca(OH)2
= 2 Ca.C6H5COO.OH
sodium benzoate:
C7H5NaO2
C6H5COONa
+ NaOH
= Na2.C6H5COO.OH
This basic salt decomposes similar to the calcium salt:
Na2.C6H5COO.OH = Na2CO3 + C6H6
Thus right or wrong ?
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I have to say Organikum, you had me worried there for a while. 'conc. H2SO4' and 'decent yeilds' are terms I use all the time,
but using them together would have meant me giving an opinion on something I had no information about 
I wasn't very clear with my use of basic salts. I'll try to clarify this at the risk of overexplaining. Calcium is a 2+ ion, so it can
form basic salts, eg...
Calcium hydroxide is [Ca]2+ [OH]- [OH]-
So basic calcium benzoate would be,
[Ca]2+ [C6H5COO]- [OH]-
And should form in preference to a mixture of calcium hydroxide and neutral calcium benzoate because the mixture would have a higher pH. This is a
hunch because if neutral calcium benzoate is less soluable, this might form instead.
In the same sort of way sulphuric acid forms acid salts like [Na]+ [H]+ [SO4]2-
Sodium only has one positive charge, so it cant form basic salts. What is trying to form here is a good mechanical mixture of sodium benzoate and
sodium hydroxide to get the hydroxide and benzoate as close as possible. It will never be as good as a basic calcium benzoate (assuming my hunch is
right) but sodium hydroxide is a much stronger decarboxylating agent than calcium hydroxide so the reaction with sodium hydroxide could give better
yeilds and should start at a lower temperature.
So it would be more accurate to write,
NaC6H5COO + NaOH => Na2CO3 + C6H6
If its possible to make a solid solution of sodium benzoate and sodium hydroxide the line between a basic salt, and a mechanical mixture becomes very
blurry.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
New setup:

Glass is jsut not the right material with caustic alkali - lets try metal. 
Running:

The first picture is more for showing how the connection was made between the can and the condensor. Gladly a 1" thread fits nicely into the can
and my 29/32 grounded glass joints fit nicely into the adaptors made for coppertubing.
"Nicely" says, "with reasonable but not excessive amounts of thick Teflon-tape".

[Edited on 13-9-2004 by Organikum]
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
The solvent-can setup yielded after the can was put onto the flame directly (flamesieve was used though) about 200ml of a morning-piss yellow liquid
with a pungent odor. After redistilliation 170ml benzene was received.
(the yellow color and the smell was already described by IPN in this thread)
Ratios:
300g sodium benzoate
180g coffee-grinder grinded NaOH
Sorry no quantitative results for the NaOH/benzoate precipitate, this got a little outa control and I have now masses of this stuff, but dont know how
much benzoate per gram is in it.... 
As soon I have used this up I will make a more controlled run of this.
ATTENTION!
I would striktly discourage the use of glassware for making the precipitate and for drying it. This strips emaille from pots like nothing and it also
stripped the Teflon from a pizza-pan like nothing.
Use an old iron pot, or an emailled old iron pot - afterwards it will be a iron pot without emaille anyways.... 
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
I hope thus gets not to boring......

This is the received liquid before redistillation. The lower part is water, what else.
It looks like, yes, like - eh - something else, but its discolored benzene. 
I spare you all a picture of the redistilled benzene, as waterclear liquids are not so very impressive..... 
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
I made several runs now and always got at least 120ml benzene from 300g sodium benzoate.
(the before posted 175ml referred to a run with 450g+ benzoate, sorry, I messed this up)
120ml is about 105g benzene, is about 1,35 mole.
300g sodium benzoate are about 2 mole.
This gives a molar yield of 68% thats ok for me.
Hope I didnt mess this up again, but I the pictures tell that this is not made up.
ORG
Addon:
A run with calcium hydroxide instead of grinded sodium hydroxide gave less product which was much more decolorized, thats a pity as Ca(OH)2 comes
already as a fine powder and would make the grinding unnecessary.
So I stay with my (new-old) coffeegrinder and NaOH.... 
[Edited on 18-9-2004 by Organikum]
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Not having anything else particularly interesting to do today.. I decided to fire up the FTIR and see what it thought about the products obtained from
the decomposition of NaC6H5COO + NaOH in my tests.
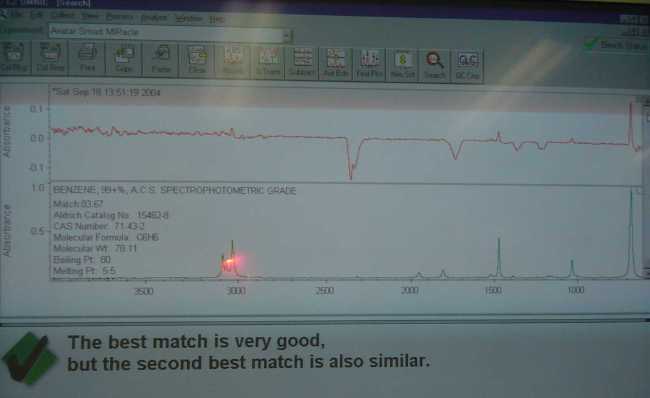
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
And the solid residue after the redistillation.
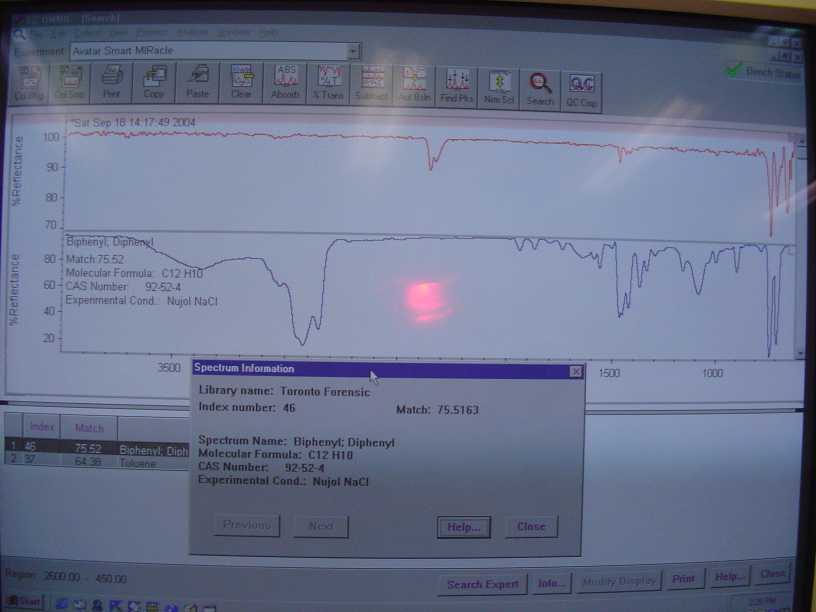
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Thanks ordenblitz, but I have no experience at all in interpreting such data, can you add some words of explanaition perhaps? Would be helpful for me
at least?
(I would guess it says the benzene is VERY good though... )
New run, better yields:
300g sodium benzoate
200g sodium hydroxide (grinded)
Result:

After IPNs and my own experiences this will give about 160ml/140g benzene after redistillation (95%), what are ~1,8 mole.
The benzoate used was ~2,1 mole.
Says ~85% molar yield, yes? 
All credits go to IPN and ordenblitz who actually DID it first and to F.C.Whack who posted the articles.
Mine is just some tinkering with the setup and the use of the industrial way of thinking.
This dry distillation business is promising, and this process is very similar to the dry distillation/condensation of the Ca or Na salts of acetic
acid and phenylacetic acid, which iyields 1-phenyl-2-propanone.
Also the making of benzaldehyde this way as mentioned by Marvin before is for sure worth a try and to invest some work into the optimization of ratios
and setup.
I propose though that the solvent-can/steelwool setup beats a flask all time here.
Sugesstion:
More professional would be to use a more robust vessel made from steel/iron and to fill it with iron or coppertubing pieces (length = 1,25xdiameter)
similar to Raschig rings.
Thats at least how I will go on.... 
[Edited on 19-9-2004 by Organikum]
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Quote:
------------------------------------------------------------------------------------
Thanks ordenblitz, but I have no experience at all in interpreting such data, can you
add some words of explanaition perhaps? Would be helpful for me at least?
------------------------------------------------------------------------------------
Bear with me as I have no actual formal training on a FTIR, what I know comes only from reading the books and playing with it for a few years. I got
this one used at an asset sale where no one except me, really knew what it was and more importantly, what it was worth. The best part was that it came
with very extensive spectral libraries. Without good libraries, the machine is only useful for comparing things you yourself have scanned. Therefore
there is no "known good" standard by which to compare unless you trust your sampling technique, conditions and materials to be as good as
the commercially available libraries.
My benzene sample for instance, was scanned and appears in the photo as the upper red spectra. I then asked the machine to search the libraries for
any matches. Luckily there were quite a few stored spectra of benzene in my libraries to compare too. What the software search function did was find
the ones that were the closest to the tested sample. The best match according to Omnic was "benzene 99+ ACS". This of course does not mean
that my sample was equivalent in quality, it simply meant that it was the best match from the available spectra in my libraries. The Aldrich library
contained three benzene, Toronto forensic had two and the commercial materials library had several industrial quality examples of C6H6, about 8 in all
to compare too. Omnic seemed to think that my sample was closer to the reagent then the industrial grade materials, but that is by no means a
certification of its quality. One other interesting feature is the "subtract option" where I can take my sample spectra and have the machine
subtract from it, the value of the ' known good ' library spectra, leaving the remainder, which in theory should be any impurities. I could
then take that impurity spectra and search the libraries to see if it finds any matches. Kind of a clever idea, but to date I really haven't
found it to work that well.
You will notice that the upper spectra contained several peaks that were obviously not in the lower library spectra. These are typically water vapor
and other gasses that are normally in the beam path of the machine. There is a distinct double peak around 2360nm on both the benzene and biphenyl
spectra I posted. It is carbon dioxide.
Usually FTIR are equipped with a N2 purge system to solve that problem, but I couldn't be bothered to hook one up. The Omnic software has several
corrections, which can be applied to account for this, and that is what I asked the machine to do when searching the libraries. There are many things
that the software search function uses to decide what weight to place on any peaks in the frequency range when comparing spectra. Unfortunately having
no idea what they are, I don't try to visually interpret the spectra, I just let the machine take it's best guess.
I hope that helps to explain what you're looking at in the above-posted pictures. I am by no means well schooled in the use of a FTIR and if
there is any one else that cares to weigh in about this interesting subject… please do!
P.S. here is a picture of the actual machine.

|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Organikum,
I really like your can…
a solvent can you called it?
I haven't seen anything like it and it looks pretty handy…
where did you get it?
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
ordenblitz,
Nice work with the spectra. Heres you being envious of the can Organikum is using when there is a thousand of us being envious about not having
access to an IR spectrometer, let alone a FTIR. Out of interest, does it need a compressed air supply?
Organikum, some nice results, but where is the water coming from? It worries me. I thought these were just runs with the materials being ground
together?
|
|
|
| Pages:
1
..
3
4
5
6
7
..
14 |