| Pages:
1
2 |
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
I have a feeling all halogen-based oxidizers will produce the nitrile and not the aldehyde. The nucleophillic nitrogen will attack the active halogen,
forming a haloamine. This will then dehydrohalogenate to a haloimine, which is stabilized against hydrolysis by the electron withdrawing halogen on
the nitrogen. Finally, this eliminates carbon dioxide and another hydrogen halide unit to form the nitrile. Note that an aldehyde can only be formed
if the haloimine intermediate hydrolyzes to the alpha keto acid, which decarboxylates as expected. This is unlikely for the reason stated above. The
usual Strecker degradation uses alloxan as the oxidizer-- this will instead form a standard imine intermediate, which will hydrolyze as we want.
Unless a large excess of the amino acid is used, I don't see how hypochlorite or TCCA could reasonably produce a good yield of aldehyde.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Cryolite.  | | I have a feeling all halogen-based oxidizers will produce the nitrile and not the aldehyde. The nucleophillic nitrogen will attack the active halogen,
forming a haloamine. This will then dehydrohalogenate to a haloimine, which is stabilized against hydrolysis by the electron withdrawing halogen on
the nitrogen. Finally, this eliminates carbon dioxide and another hydrogen halide unit to form the nitrile. Note that an aldehyde can only be formed
if the haloimine intermediate hydrolyzes to the alpha keto acid, which decarboxylates as expected. This is unlikely for the reason stated above. The
usual Strecker degradation uses alloxan as the oxidizer-- this will instead form a standard imine intermediate, which will hydrolyze as we want.
Unless a large excess of the amino acid is used, I don't see how hypochlorite or TCCA could reasonably produce a good yield of aldehyde.
|
That very well may be correct, but there are references on this page which state otherwise: http://www.sciencemadness.org/talk/viewthread.php?tid=66331
I have tried this before, and while I never managed to obtain a yield that I could measure, the rose smell was powerful and unmistakable.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Amos  | Quote: Originally posted by JJay  |
That's interesting. I have 20 grams of phenylalanine, but I was kind of planning on using it to try to make phenylacetaldehyde by hypochlorite
oxidation and was unaware that any benzyl cyanide might be produced... do you have a literature reference for this? |
JJay, I tried the same thing hoping to get phenylacetaldehyde; I just treated a solution of phenylalanine in water with a large excess of concentrated
bleach with periodic cooling, and based on the resulting product and its odor, assumed it was the aldehyde. Until I shot in on the GC-MS at work and
discovered the following chromatogram: |
Wow, that's pretty amazing. That would explain why I never managed to isolate any product....
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Amos, that spectra is really interesting, it shows that you are getting aromatic halogenation to a limited extent (c 10%). Maybe using a slight
excess of phenylalanine and keeping the solution cool would help to reduce this side reaction. Its certainly a problem to bear in mind. When I use
hypochlorite solutions I usually standardize it against iodide/thiosulphate so that I know what I am dealing with.
@Cryolite, I haven't read the aldehyde papers but I would have thought that you could simply use hald the amount of hypochlorite /TCCA and then
hydrolyse the imine as a sequential process, I doubt that you would even have to isolate the imine.
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
@Boffis: I'm pretty sure that using stoichiometric amounts of TCCA would at best lead to a mixture of starting material, aldehyde, and nitrile. You
may actually do worse than this, as imines are less basic than amines and so are likely to be more able to get halogenated, especially without excess
base. Then again, I haven't done the reaction and I'm just theorizing things, so I might be wrong.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
@Cryolite: As JJay pointed out, there are numerous examples in the literature of hypochlorite being used to convert amino acids into
aldehydes, so it would seem that it is indeed possible. For example, the authors of one of the references in the thread JJay linked to claimed to have
obtained phenylacetaldehyde from phenylalanine in 60% yield using one equivalent of sodium hypochlorite in aqueous solution. It should be noted,
however, that both of their amino acid and sodium hypochlorite solutions were extremely diluted, and a phosphate buffer was used to maintain a pH of
around 7. It's also worth mentioning that the hypochlorite solution was added very slowly over the course of ten minutes with constant stirring.
I think the reason Amos failed to produce any phenylacetaldehyde was because he used a large excess of concentrated bleach. I believe the key to
getting the aldehyde is keeping the concentration of the hypochlorite as low as possible, while also keeping the pH as close to neutral as possible.
The idea here is to chlorinate the nitrogen once and then quickly eliminate the chloro group via decarboxylation. We want to avoid forming an imine
through normal E2 elimination prior to the decarboxylation step. By keeping the hypochlorite concentration low, we avoid over-chlorinating the
nitrogen; by keeping the pH neutral, we avoid eliminating the chloro group via deprotonation of the adjacent carbon. Also, by keeping the pH closer to
neutral, most of the hypochlorite will be in the form of hypochlorous acid, which is most likely the real oxidizing species in these reactions anyway.
In Amos's case, the large stoichiometric excess of hypochlorite most likely led to over-chlorination and too high of a pH, favoring the nitrile over
the aldehyde.
The reaction we want is this:
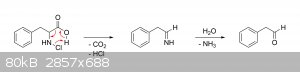
The reaction we don't want is this:
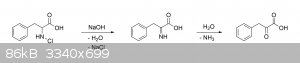
The problem with forming the imine before the decarboxylation step is that even if the imine hydrolyzes immediately afterwards, the carboxyl group
can't just suddenly leave as CO2 now that it's an alpha-keto acid. Just like in Strecker degradations, something must first act as an
electron sink before the carboxyl group can leave, otherwise you inevitably end up with an extremely unfavorable acyl anion intermediate, which are
highly unstable and rarely ever observed in practice (if at all).
While I suppose it's possible that an alpha-keto acid intermediate may actually help to facilitate the decarboxylation of unreacted amino acid
molecules by condensing with them and acting as an electron sink, the problem is that the reaction mixture is not only aqueous, but extremely dilute
as well. So how beneficial this would turn out to be, I don't know. My guess is that something like this would have to happen in order for
decarboxylation to occur:
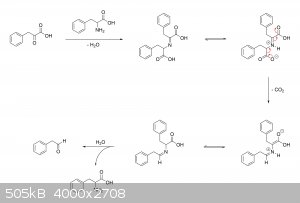
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
If the pH is too high like it is usually in commercial bleach for safety reasons (avoid to set toxic gaseous Cl2 free), you will get nitrile and
subsequent hydrolysis products (phenylethanoic acid, phenylethanoic amide and NH3), but also aldehyd crotonisation/condensation of phenylethanal from
the very activated benzyl position (trapped between the aromatic EWG and the aldehyd EWG...both helping also terminal decarboxylation.
You will end up with a polymer (-CH(Ar)-CHOH-)n that will crotonise to (-CH(Ar)=CH-)n + n H2O thus poly-phenylacetylen and eventually the cyclic
trimer 1,3,5-triphenyl-benzene.
[Edited on 16-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by JJay  | Quote: Originally posted by Amos  |
JJay, I tried the same thing hoping to get phenylacetaldehyde; I just treated a solution of phenylalanine in water with a large excess of concentrated
bleach with periodic cooling, and based on the resulting product and its odor, assumed it was the aldehyde. Until I shot in on the GC-MS at work and
discovered the following chromatogram: |
Wow, that's pretty amazing. That would explain why I never managed to isolate any product.... |
I thought for sure I had the correct product too, as a general trend is that these large aromatic nitrile molecules tend to smell very similar to
their corresponding aldehydes; so much so that scented consumer products meant for more robust environments (such as detergents and cleaners) often
substitute a nitrile for more reactive aldehyde aroma compounds (cinnamaldehyde, citral, etc.)
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by Boffis  | | @Amos, that spectra is really interesting, it shows that you are getting aromatic halogenation to a limited extent (c 10%). Maybe using a slight
excess of phenylalanine and keeping the solution cool would help to reduce this side reaction. Its certainly a problem to bear in mind. When I use
hypochlorite solutions I usually standardize it against iodide/thiosulphate so that I know what I am dealing with. |
Oh, I know I definitely went overboard; I also left the product sitting underneath the bleach for far too long. This is something I intend to carry
out in a more controlled manner at a later date and provide a write-up for on the forum.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Amos  | Quote: Originally posted by JJay  | Quote: Originally posted by Amos  |
JJay, I tried the same thing hoping to get phenylacetaldehyde; I just treated a solution of phenylalanine in water with a large excess of concentrated
bleach with periodic cooling, and based on the resulting product and its odor, assumed it was the aldehyde. Until I shot in on the GC-MS at work and
discovered the following chromatogram: |
Wow, that's pretty amazing. That would explain why I never managed to isolate any product.... |
I thought for sure I had the correct product too, as a general trend is that these large aromatic nitrile molecules tend to smell very similar to
their corresponding aldehydes; so much so that scented consumer products meant for more robust environments (such as detergents and cleaners) often
substitute a nitrile for more reactive aldehyde aroma compounds (cinnamaldehyde, citral, etc.) |
I probably won't be trying it again. The last thing I need is to accidentally manufacture a listed chemical....
|
|
|
Mush
National Hazard
   
Posts: 632
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
Odorous Products of the Chlorination of Phenylalanine in Water: Formation, Evolution, and Quantification
Ingrid FreuzeStéphan BrosillonDorine HermanAlain LaplancheChristian DémocrateJacques Cavard
Environ. Sci. Technol.2004; 38, 15, 4134-4139
Publication Date:June 22, 2004
https://doi.org/10.1021/es035021i
Abstract
To explain some of the possible origins of an odor episode which took place in a drinking water supply in the region of Paris (France), the
chlorination reaction in water of phenylalanine was studied. This amino acid was chosen for first experiments because of its physical and chemical
particular properties. Changes in the different byproducts formed were followed by high-performance liquid chromatography (HPLC) over a period of
time. N-chlorophenylalanine (mono-N-chlorinated amino acid) and then phenylacetaldehyde were the major products formed for the lower chlorine to
nitrogen molar ratios. For Cl/N molar ratios of 1 and beyond, phenylacetonitrile and N-chlorophenylacetaldimine appeared and increased with the
chlorination level. N-chlorophenylacetaldimine was quantified by using its difference of stability in various organic solvents. Our attention was
first directed to the monochlorinated derivative but further examination indicated that it could not be responsible for odor troubles: it
dissociated before reaching the consumer's tap and it was produced at consistently low yields under conditions relevant to drinking water treatment.
On the contrary, chloroaldimine appeared to be a very odorous and water-stable product: it strongly smells of swimming pool with a floral
background. The odor detection threshold is about 3 μg·L-1 and it can persist for more than one week at 18 °C. It is now suspected of being a
source of off-flavor concerns among consumers.
http://sci-hub.tw/10.1021/es035021i
|
|
|
| Pages:
1
2 |