Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
Need help on amino alcohols
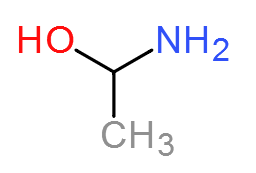
I am currently trying to synthisize 1Amino-1ethanol to be chlorinated so I can use it in a grignard by Chlorinating the OH group. Unfortunately I have
no idea how to synthesize it. I am thinking adding ammonia to acetyl Chloride, then using LAH to reduce the =O back into an OH group. However I would
really like to avoid LAH if I can. Any help and thoughts is appreciated!
I have an image of the molecule I am after below. (note: I know I spelled ethanol wrong! It is 2 AM when I am posting this.)
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
That compound would be unstable at STP. It would give off ammonia to form acetaldehyde.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
And the ammonia would react with the acetaldehyde to make an imine.
And, if you could make it, you couldn't turn it into a Grignard reagent because of the amino group.
And if you could get that stuff and convert the OH to Cl, the chloro amine would polymerise into a mess.
What are you actually trying to make?
Because there's got to be a better way to do it.
|
|
|
Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
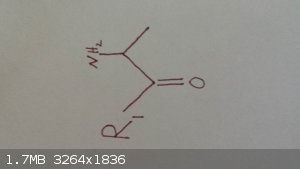
This is the molecule I am ultimately after. R1 is a straight chain alkane.
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
There are many perfectly good alpha-amination reactions, consider using them. This nucleophilic amine addition of yours doesn't work as previously
stated. The motif looks awfully much like it belongs to phenethylamine class, though...
[Edited on 1-9-2014 by kavu]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
If you're not after chirality, perhaps you could add nitroethane to an appropriate acyl(+) synthon. Reduction of the nitro group to an amine should be
straightforward.
|
|
|
Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
Got it thank you. And kavu, in case you were wondering I am not after anything that would really fall into a phenylethylamine (drug) class of
molecule. Unfortunately we live in a day where as soon as you even mention synthesizing something with the words "phenyl" and "amine" you get a lot of
suspicion of being a meth cook in some turgid lab in the basement of some dilapidated house. Shame.
I am going to be atraching a butane group in for R1 and I was going to attach diphenylmethanol in on the ketone group. However like was mentioned
before grignard do not work with compounds that have amines in them.
DJF90, I will probably gave do do just that and will have to take care of attaching the diphenyl group before dealing with amination.
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Knoevanagel condensation between valeraldehyde (pentanal) and nitroethane will give a nitroalkene which can undergo michael addition with Ph2CH(-) (I
assume this is what you meant by "diphenylmethanol group). Reduction of the aliphatic nitro group then gives the desired product (which you've not
actually drawn...)
Because the product of the Michael addition is more acidic than the Ph2CH(-), you'll have to take care which way around you do the addition and use an
excess of the appropriate reagent (but I'll leave you to work these finer details out).
[Edited on 1-9-2014 by DJF90]
|
|
|